Figure 4.
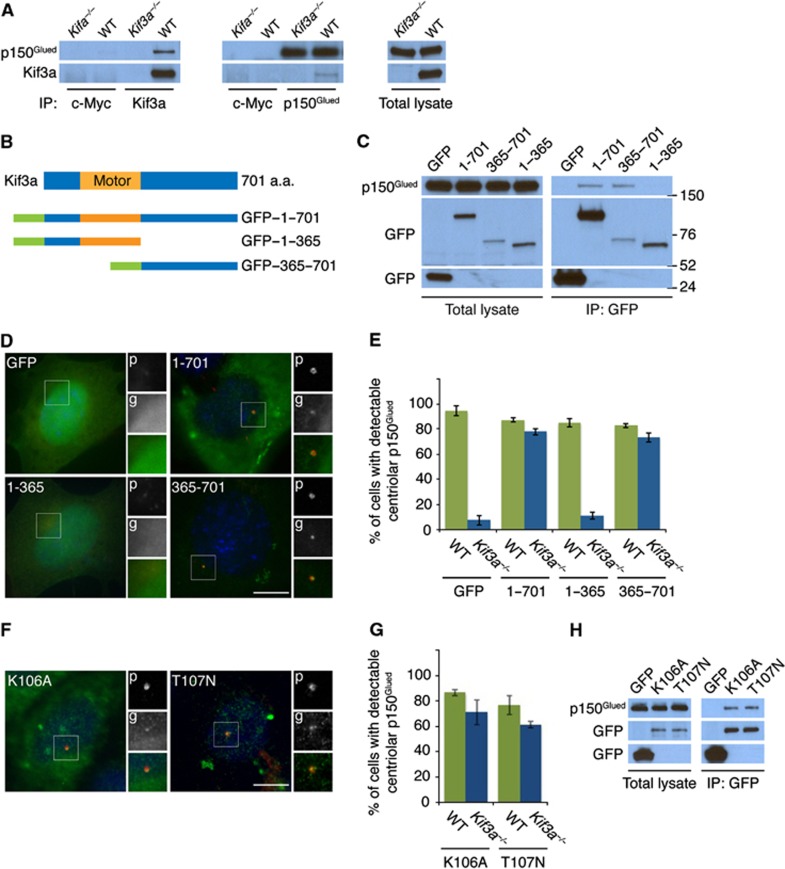
Kif3a is required to localize p150Glued to the subdistal appendage. (A) WT and Kif3a−/− total cell lysates were subjected to immunoprecipitation of Kif3a, p150Glued and c-Myc, which served as a negative control. Precipitating proteins were subjected to immunoblotting for Kif3a and p150Glued. (B) Schematic representations of the motor domain and the truncation mutants of GFP-tagged Kif3a. (C) We expressed the indicated GFP-tagged versions of Kif3a in HeLa cells and immunoprecipitated GFP. Endogenous p150Glued and each of the Kif3a fusion proteins were detected by immunoblotting for p150Glued and GFP, respectively. (D) Expression of the full-length GFP-tagged Kif3a and the truncated mutant that interacted with p150Glued (‘g’, green) restored the localization of p150Glued (‘p’, red) to the subdistal appendage in Kif3a−/− cells. (E) Quantification of p150Glued localization to the subdistal appendage. (F) Expression of the motor dead GFP-tagged Kif3a (K106A and T107N) mutants ('g', green) restored the localization of p150Glued (‘p’, red) to the subdistal appendage of Kif3a−/− MEFs. (G) Quantification of p150Glued localization to the subdistal appendage in Kif3a mutants. (H) We immunoprecipitated the GFP tag of the motor dead Kif3a forms and detected endogenous p150Glued and GFP. At least 100 cells were analysed per experiment (n=3), P<0.001 (paired t-test). Scale bars indicate 5 μm for all images.
Source data for this figure is available on the online supplementary information page.