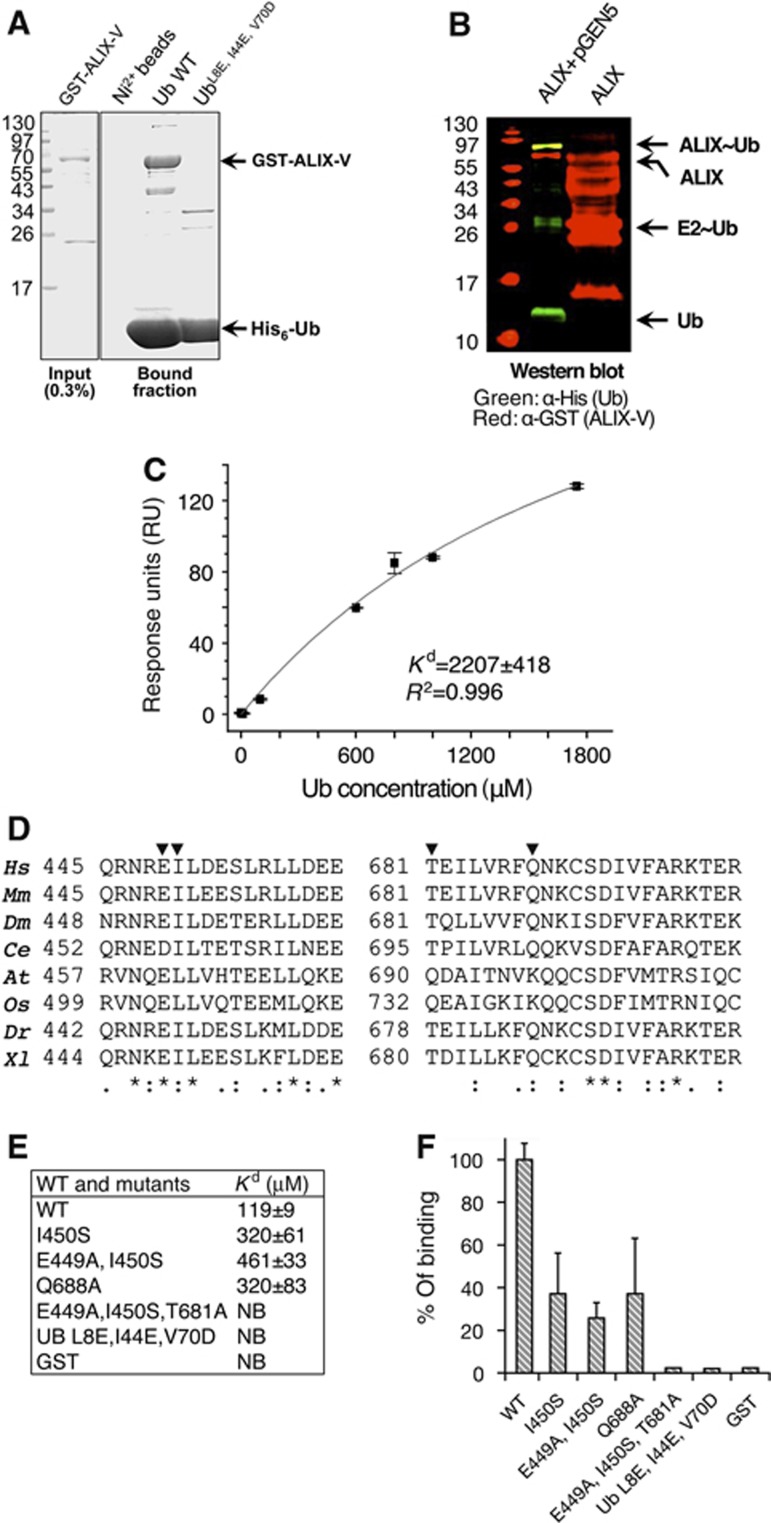
Figure 4.
ALIX-V binds Ub through the Ub I44 hydrophobic patch. (A) Shows Coomassie stained SDS–PAGE of pull-down assay; bacterial recombinant His6-Ub and Ub mutant immobilized to Ni2+-shepharose were used in a pull-down with E. coli lysate expressing pGST-ALIX-V. (B) Shows ALIX-V ubiquitylation in bacteria. Bacterial lysates co-expressing His6-Ub, Uba1, yeast Ubc5 (expressed from pGEN5) and GST-ALIX-V were purified on Ni2+ sepharose beads and subjected to western blot analysis. Only in the presence of the ubiquitylation machinery components, a band corresponding to mono-ubiquitylated ALIX-V is shown (yellow) as detected by both α-GST (red) and α-His6 (green) antibodies (C) SPR binding affinities measured for GST-ALIX-V with Ub. GST-ALIX-V was immobilized using α-GST antibody and binding affinity was measured with increasing concentrations of Ub. Data were analysed and fitted using the Origin software with single-binding site model. (D) Conservation of the Ub-binding patch within species (generated by ClustalW). Multiple sequence alignment of regions within the binding patch is shown. Triangles indicate residues that were mutated. Conservation is shown at the bottom of the alignment: (*)—full conservation, (:)—strongly similar residues and (.)—weakly similar residues. (E–F) Affinity measurements of ALIX-V:Ub WT and mutant proteins. Binding affinity measurements between GST-ALIX-V and Ub proteins were determined by MST. At, Arabidopsis thaliana; Ce, Caenorhabditis elegans; Dm, Drosophila melanogaster; Dr, Danio rerio; Hs, Homo sapiens; Mm, Mus musculus; Os, Oryza sativa; Xl, Xenopus laevis.