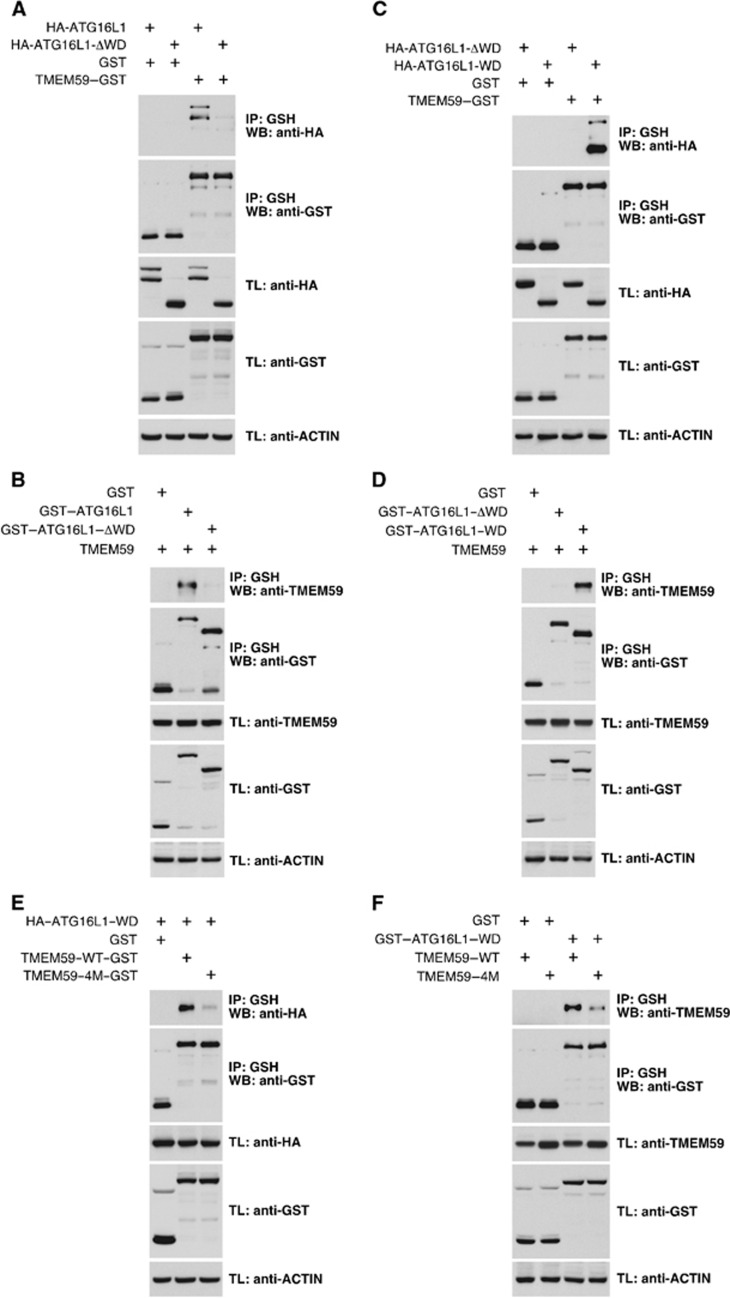
Figure 7.
The ATG16L1-binding motif present in TMEM59 recognizes the WD-repeat domain of ATG16L1. (A–F) 293T cells were transfected with the indicated constructs, lysed and subjected to GST immunoprecipitation with agarose beads coupled to glutathione. Shown are WBs against the indicated molecules. (A) A deleted version of ATG16L1 lacking the WD domain (HA–ATG16L1–ΔWD) does not co-precipitate with TMEM59–GST. (B) TMEM59 does not co-precipitate with ATG16L1–ΔWD fused to GST. (C) The WD domain of ATG16L1 (HA–ATG16L1–WD) suffices to co-precipitate with TMEM59–GST. (D) TMEM59 co-precipitates with ATG16L1–WD fused to GST. (E) HA–ATG16L1–WD does not bind a 4M version of TMEM59–GST. (F) TMEM59–4M does not co-precipitate with the ATG16L1–WD fused to GST.
Source data for this figure is available on the online supplementary information page.