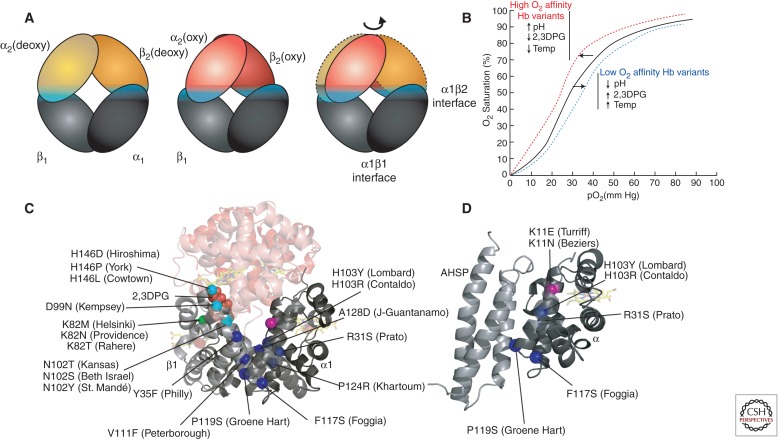
Figure 2.
Hb variants with altered subunit interactions. (A) Conversion from the low O2 affinity (deoxy, T state) to high O2 affinity (oxy, R state) involves a relative rotation of the α1β1 and α2β2 dimers, with changes in contacts across the α1β2 (and α2β1) interface (cyan). In this cartoon, the α1β1 dimer is held stationary to reveal the relative motion of the α2β2 dimer in going from the deoxy (orange) to the oxy (red) states. (B) The sigmoidal shape of the Hb-O2 saturation curve shows allosteric regulation by changes in pH, temperature, and 2,3DPG. These regulators, as well as Hb variants, influence the shape of the curve. High oxygen affinity variants, high pH, low 2,3DPG, or low temperature induce a “left shift” in the saturation curve (red line). Conversely, low oxygen affinity variants, low pH, high 2,3DPG, or high temperature induce a “right shift” (blue line). (C) Hb sequence variants at the allosteric α1β2 interface (cyan spheres) show an impaired response to O2 binding. Some sequence variants disrupt binding to other allosteric regulators, e.g., substitutions at βK82 (green) disrupt interactions with 2,3DPG that normally stabilize the low O2 affinity T state. Mutations that disrupt α1β1 (and α2β2) dimerization (blue spheres) increase the concentration of free monomers, which are unstable. (D) Some α mutations that disrupt binding to β may also disturb binding to the chaperone, AHSP. Other α variants, such as Turriff and Beziers (pink sphere) may inhibit only AHSP binding.