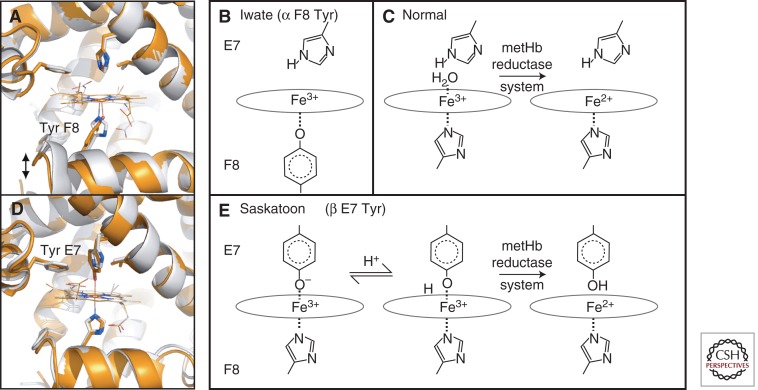
Figure 4.
Examples of M-type hemoglobins. (A) The heme pocket of wild-type Mb (gray) and the F8 His > Tyr mutation (orange, PDB 1HRM), which serves as a model for Hb M-Iwate. Effects on the protein fold are to increase the distance from the heme to the F helix, recapitulating features of the deoxy-α (T state) structure. (B) In Hb M-Iwate, α 87 Tyr F8 is deprotonated and favors Fe3+ oxidation state, resulting in rapid autoxidation. This ferric form is not reduced by met-Hb reductase. (C) With the normal His F8 present, ferric heme is readily reduced. (D) Substituting the distal His E7 side chain in Mb for a larger Tyr E7 (orange, PDB 1MGN), as also occurs in Hb Saskatoon, brings the Tyr hydroxyl group within binding distance of the iron, forming a hexacoordinate iron site. (E) In Hb Saskatoon the hexacoordinate ferric iron in the effected β chains can be reduced by met-Hb reductase, possibly owing to a transient protonation of Tyr E7. Note that Mb is used as a model for Hb subunits in A and D, whereas B and E are based on Hb spectroscopy.