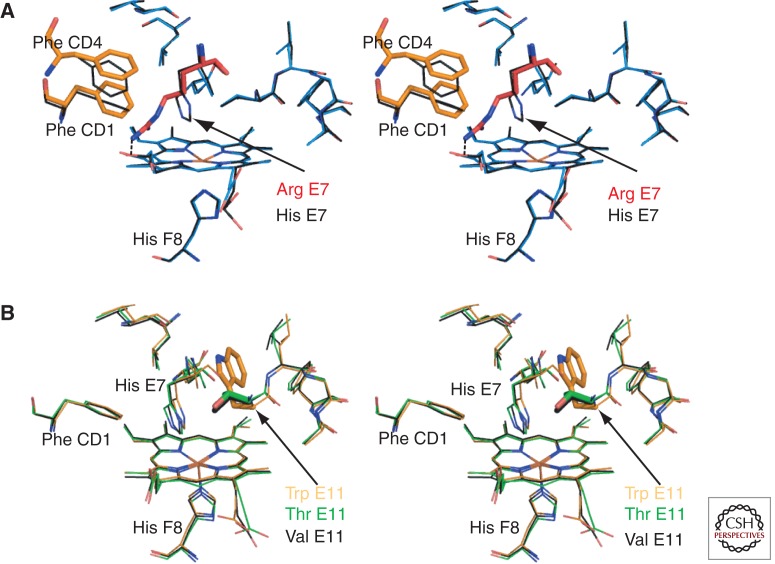
Figure 5.
Hb variants with amino acid changes in the heme pocket. (A) Stereo diagram of a model of the deoxy Hb Zurich β heme pocket (blue) overlaid with the wild-type β heme pocket (black, PDB 2DN2). This model of Hb Zurich is based on the structures solved by Phillips et al. (1981) and Tucker et al. (1978). It was generated with the macromolecular modeling program Coot (Emsley et al. 2010) by mutating the distal His of deoxy-β (PDB 2DN2) to Arg. The distal Arg E7 (red) is oriented toward the CD corner, disturbing the position of Phe CD1 and Phe CD4 (orange). The heme pocket entrance is much wider allowing increased access to ligands. However, unlike normal His E7, mutant Arg is unable to stabilize bound O2 via hydrogen bonding. (B) Stereo diagram showing the structural changes associated with substitution of β Val E11. The wild-type structure carrying the branched hydrophobic side chain Val (black bonds, PDB 2DN2) is overlaid with structures carrying the largest aromatic side chain Trp E11 (orange, PDB 101K) or a polar side chain Thr E11 (green PDB, 1HDB). It is clear that no major backbone or side chain repacking occurs. Thus, mutations in this position are likely to have specific effects in changing the volume of the heme pocket accessible to solvent or diatomic ligands and the electrostatic properties of the pocket. These changes will manifest as differences in O2 binding, ligand selectivity, autoxidation, and heme loss.