Abstract
Aim
The effects of atomoxetine (20 and 60 mg twice daily), 400 mg moxifloxacin and placebo on QTc in 131 healthy CYP2D6 poor metabolizer males were compared.
Methods
Atomoxetine doses were selected to result in plasma concentrations that approximated expected plasma concentrations at both the maximum recommended dose and at a supratherapeutic dose in CYP2D6 extensive metabolizers. Ten second electrocardiograms were obtained for time-matched baseline on days −2 and −1, three time points after dosing on day 1 for moxifloxacin and five time points on day 7 for atomoxetine and placebo. Maximum mean placebo-subtracted change from baseline model-corrected QT (QTcM) on day 7 was the primary endpoint.
Results
QTcM differences for atomoxetine 20 and 60 mg twice daily were 0.5 ms (upper bound of the one-sided 95% confidence interval 2.2 ms) and 4.2 ms (upper bound of the one-sided 95% confidence interval 6.0 ms), respectively. As plasma concentration of atomoxetine increased, a statistically significant increase in QTc was observed. The moxifloxacin difference from placebo met the a priori definition of non-inferiority. Maximum mean placebo-subtracted change from baseline QTcM for moxifloxacin was 4.8 ms and this difference was statistically significant. Moxifloxacin plasma concentrations were below the concentrations expected from the literature. However, the slope of the plasma concentration−QTc change observed was consistent with the literature.
Conclusion
Atomoxetine was not associated with a clinically significant change in QTc. However, a statistically significant increase in QTc was associated with increasing plasma concentrations.
Keywords: atomoxetine, ICH E14, moxifloxacin, QTc, thorough QT
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Prior to this study, estimates of the influence of atomoxetine on the heart rate corrected QT interval (QTc) were based on the routine clinical population entered into clinical trials treated with a variety of doses as clinically appropriate.
WHAT THIS STUDY ADDS
This manuscript informs the clinical community with regards to the potential influence of atomoxetine on QTc at maximum exposure (CYP2D6 subjects treated with the maximum approved dose in a TQT study).
Introduction
Atomoxetine is a potent and selective inhibitor of the norepinephrine transporter used to treat attention deficit hyperactivity disorder. Atomoxetine underwent clinical development prior to the introduction of the ICH E14 Guidelines 1. Therefore, ‘a thorough QT (TQT) study’ was not conducted. The pharmacology of atomoxetine relevant to cardiac depolarization and repolarization is complex. The IC50 for blockade of the IKr potassium channel (hERG) has been reported as 0.869 μm (IC20 of 0.067 μm) (unpublished data on file, Department of Toxicology, Eli Lilly and Company, Indianapolis, IN, USA, Lundeen_Gregg_R@lilly.com) and 6.3 μm [2] without rate dependency in either study. Such diversity of results in hERG assays is not uncommon [3]. The IC50 for blockade of hERG by the two primary metabolites of atomoxetine, 4-hydroxyatomoxetine and N-desmethylatomoxetine, as determined in the same study that found an IC50 for atomoxetine of 0.869 μm, was 20.0 μm and 5.71 μm, respectively (unpublished data on file, Department of Toxicology, Eli Lilly and Company, Indianapolis, IN, USA, Lundeen_Gregg_R@lilly.com). Atomoxetine is metabolized primarily by the polymorphically expressed enzyme cytochrome P450 2D6 (CYP2D6). Therefore, there is a population of CYP2D6 extensive metabolizers (EMs) distinct from the smaller population of CYP2D6 poor metabolizers (PMs) with the plasma half-life of atomoxetine ∼21 h in PMs vs. ∼5 h in EMs [4]. Total Cmax for 4-hydroxyatomoxetine and N-desmethylatomoxetine is approximately 0.1% and 45%, respectively in PM subjects (unpublished data on file, Department of Clinical Pharmacology, Eli Lilly and Company, Indianapolis, IN, USA, Mitchell_Malcolm_I@lilly.com). Consequently, the average steady-state concentrations of atomoxetine are approximately 10-fold higher in PMs compared with EMs. The clearance of atomoxetine in EM children is 1.24-fold greater than the clearance in EM adults (unpublished data on file, Department of Clinical Pharmacology, Eli Lilly and Company, Indianapolis, IN, USA, Mitchell_Malcolm_I@lilly.com). Furthermore, in PM children it is 9-fold lower than in EM children while in PM adults the clearance is 10-fold lower than in PM adults (unpublished data on file, Department of Clinical Pharmacology, Eli Lilly and Company, Indianapolis, IN, USA, Mitchell_Malcolm_I@lilly.com) and therefore adult PM subjects achieve more than adequate exposure relative to children. The highest anticipated exposure to atomoxetine should occur in PMs at the maximum approved daily dose of 1.8 mg kg day with the absolute maximum dose of 100 mg day regardless of body weight. A high dose of atomoxetine 60 mg twice daily was evaluated in the adult subjects in this study. In adult PM subjects at the 60 mg twice daily dose, the maximum Cmax plasma concentration observed has been 5016 ng ml (unpublished data on file, Department of Clinical Pharmacology, Eli Lilly and Company, Indianapolis, IN, USA, Mitchell_Malcolm_I@lilly.com). Atomoxetine is approximately 98% protein bound [5], and therefore peak free plasma concentrations might reach 0.34 μm. Given such free plasma concentrations at the extreme upper end of observed values in adult PM subjects treated with 60 mg twice daily, some delay in ventricular repolarization and increase in the corrected QT interval (QTc) might be expected based on the IC20 value of 0.067 μm and the IC50 value of 0.869 μm (unpublished data on file, Department of Toxicology, Eli Lilly and Company, Indianapolis, IN, USA, Lundeen_Gregg_R@lilly.com). This free plasma concentration could result in greater than 20% hERG blockade [6, 7] and there would be <30-fold margin (actual margin of 2.6-fold) between this free plasma concentration and the IC50 concentration [6]. However, atomoxetine also blocks both the sodium INa and calcium ICa channels with IC50 values of 36.1 μm and 1.93 μm, respectively (unpublished data on file, Department of Toxicology, Eli Lilly and Company, Indianapolis, IN, USA, Lundeen_Gregg_R@lilly.com). Blockade of the INa channel is both rate and voltage dependent, and blockade of these channels could act to oppose depolarization prolongation.
At a concentration of 10 μm, the compound reduced the action potential duration (APD95) by 21%, decreased Vmax by 54%, and decreased the action potential amplitude by 12% in the canine Purkinje assay (unpublished data on file, Department of Toxicology, Eli Lilly and Company, Indianapolis, IN, USA, Lundeen_Gregg_R@lilly.com). However, in guinea pig cardiomyocytes, atomoxetine at concentrations of both 1 and 3 μm significantly prolongs APD20, APD50 and APD90 with the greatest effect on APD20 [2]. Discrepancies between the canine Purkinje assay and the guinea pig cardiomyocyte assay results have been described and might relate to mixed channel blockade effects [8].
A pooled analysis from acute phase patient studies detected an association between atomoxetine and statistically significant QTc prolongation in adult patients using the Bazett correction (difference +5.7 ms). However, using the Fridericia correction or a formula based on the baseline data for the specific study population (QTc = QT/RR0.39) no association between atomoxetine and QTc prolongation was detected [9]. With both of the latter correction methods, the difference between atomoxetine and placebo was actually negative [9]. Among such population-based correction formulae, the Fridericia correction formula is probably the most appropriate for correcting atomoxetine data because atomoxetine treatment increases heart rate significantly. However, a limitation in this published analysis is that it did not specifically consider only PM patients dosed at the highest approved dose of 60 mg twice daily.
Three cases of QTc interval prolongation associated with atomoxetine overdose have been independently reported. A 15-year-old who was concomitantly treated with bupropion, risperidone, alprazolam and atomoxetine took an overdose of atomoxetine only (1200 mg) and was reported to have a QTc of 607 ms (heart rate in the 110 beats min range, QT correction method not reported) [10]. A 19-year-old who was concomitantly treated with paroxetine, albuterol, montelukast, oxcarbazepine, quetiapine and atomoxetine overdosed on atomoxetine 1200 mg, oxcarbazepine 600 mg and quetiapine 9000 mg. His initial electrocardiogram (ECG) was reported as normal sinus rhythm with a rate of 99 beats min, QT interval of 397 ms and QTc of 483 ms (QT correction method not reported and the reported QTc was between values that would result from Bazett and Fridericia correction formulae) [11]. The concomitant medications for these first two patients included drugs that are inhibitors of CYP2D6 metabolism and were confounded by co-ingestants that could have influenced QTc. A 17-year-old, treated with no other medications, took an overdose of atomoxetine alone (2840 mg) and was reported to have a QTc of 476 ms with sinus tachycardia of 103 beats per min (correction method not reported but value consistent with Bazett method) [12].
Given the complex pharmacology of atomoxetine relative to cardiac ventricular depolarization and repolarization, along with the possibility that atomoxetine might result in delayed repolarization in overdose, a TQT study with atomoxetine was appropriate to conduct at maximum expected plasma concentrations (maximum approved therapeutic administration but with metabolic inhibition). With atomoxetine, rather than testing with multiples of the maximum approved dose, an alternative strategy was possible. The maximum approved dose on a weight adjusted basis (greater than maximum approved dose on an absolute dose basis) could be administered to a population of exclusively adult PM subjects. In such subjects, the addition of a metabolic inhibitor as a concomitant medication in clinical practice does not result in further increase in atomoxetine plasma concentration.
Methods
Study design
This was a phase I, multicentre, four-period crossover, inpatient study in healthy PM males conducted between March 2008 and March 2009. The primary objective was to determine if atomoxetine, at maximum expected concentrations in PM subjects at 120 mg day, was not inferior to placebo in the maximum time-matched mean difference in change from baseline in QTc [the upper bound of the one-sided 95% confidence interval (CI) <10 ms]. Subjects were randomly assigned to one of four sequence groups based on a Williams design [13] (Figure 1). Treatments were atomoxetine (20 and 60 mg twice daily), placebo, and a single 400 mg dose of moxifloxacin. The 60 mg twice daily dose in PM subjects would result in the highest atomoxetine exposure possible under approved labelling. The 20 mg twice daily dose was expected to produce atomoxetine exposure in the PM subjects that would be approximately equivalent to the exposure resulting from a 60 mg twice daily dose in EM subjects. To reach the 60 mg twice daily dose, atomoxetine was up-titrated for 2 days before reaching the 60 mg twice daily dose and subjects were expected to reach steady-state exposure by day 7 of treatment. The 20 mg twice daily dose was initiated on the first day of treatment. Subjects and investigators were blinded to atomoxetine and placebo. Moxifloxacin was branded Avelox given open label.
Figure 1.
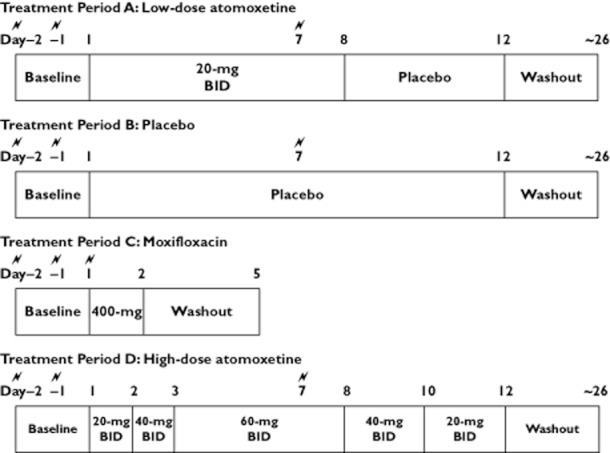
Study design.  = electrocardiogram for QTc assessment; BID = twice daily dosing
= electrocardiogram for QTc assessment; BID = twice daily dosing
Subjects took morning doses between 08.00 h and 10.00 h, 2 h after consuming a standardized breakfast. Each subject received the doses at the same time across all treatments. Morning and evening doses were separated by about 12 h. Up titration was used with the 60 mg twice daily dose of atomoxetine to mitigate potential adverse events that occur at high exposures. Because of the potential for withdrawal effects on abrupt discontinuation from high dose atomoxetine in healthy volunters, tapered discontinuation was used. Subjects resided at the clinical research unit during treatment periods and were discharged during the washout periods between treatments.
Subjects
Subjects were recruited at three centres in South Africa. Each subject gave informed consent. The protocol was approved by the Ethics Committee of the Faculty of Health Sciences Internal, an independent ethics committee at the University of the Free State, Bloemfontein, South Africa. The study was implemented in accordance with the Declaration of Helsinki and Good Clinical Practice.
Healthy PM males (age range 18–60 years) with a body weight of 50–90 kg (± 5%) were enrolled. PM status was verified by xTAG™ Mutation Detection Kit CYP450-2D6 Variants. Subjects were excluded if their ECGs showed evidence of first, second or third degree heart block, abnormal widening of the QRS complex, or other findings that would complicate QT interval measurement, or if they had cardiovascular disorders (myocardial infarction, stroke, deep vein thrombosis), personal or family history of long QT syndrome, or family history of sudden death. Strenuous physical activity, beverages and foods containing xanthines, prescription medications and herbal products 2 weeks before the first treatment, over the counter medications 1 week before the first treatment, and alcohol 2 days before or during the study periods were prohibited. Smokers were advised to keep their habit the same during the study, particularly the amount and timing of cigarette use relative to the ECG measurements. Subjects were to refrain from smoking for approximately 2 h prior to each ECG.
Electrocardiogram assessment
Before each dosing period, subjects underwent a 2 day baseline ECG assessment using the same collection time points as on treatment days. The 2 baseline days were a means of accounting for potential inter-day variability [14] and to provide for collection of a total of 405 ECGs to be used in computation of individual correction factors, as Couderc and colleagues [15] have suggested that about 400 ECGs are required for computation of stable and reliable individual correction factors.
On day 1 of the moxifloxacin period and on day 7 of the atomoxetine and placebo periods, ECGs were collected 1, 2, 4, 6 and 12 h after the morning dose. These time points were expected to encompass both the tmax of atomoxetine and times that QTc prolongation has been observed with moxifloxacin.
Subjects rested for at least 5 min prior to ECG recording. Nine replicate ECGs were recorded at approximately 1 min intervals, with subjects awake in the supine position. All ECG interval measurements presented and discussed are signal-averaged values from the nine replicates. ECGs were collected electronically on Eli250 machines (Mortara Instruments, Milwaukee, WI, USA), transmitted to the Mortara E-Scribe data management system located at a central interpretive core laboratory (COResearch, Durham, NC, USA), and over-read and measured at the central interpretive core laboratory. A total of seven over-reading cardiologists were used for the over-read of the ECGs collected in this study, but the same cardiologist was used to over-read all of the ECGs for a given subject. The QT interval was measured using the superimposed median beat derived from the full 10 s of all 12 leads. The Mortara Veritas algorithm derived the median beat. Final review and overread measurement was performed on-line using AMPS CAL ECG analysis software. The cardiologist would review the computer-placed annotations for every ECG and verify or adjust the annotations as needed on-line using electronic calipers. The QRS was defined as extending from the earliest ventricular depolarization to the offset of ventricular depolarization (J-point). The QT was defined as from the beginning of the QRS interval (earliest ventricular depolarization) to the T offset (the intersection of the terminal portion of the T wave and the isoelectric line following the T wave). The U wave, if present, was ignored. If a U wave or abnormal T wave obscured the offset of the T wave, then the offset of the QT interval was defined as the terminal portion of the T wave and the isoelectric baseline. To determine the average RR value for the ECG, the RR for 3 beats that were within 100 ms of each other were annotated, and this value was averaged to derive the average RR for the 10 s ECG.
QT correction method
In order to avoid potential difficulties with the individual QTc correction method under the circumstance where heart rates are substantially different during treatment compared with those during lead-in periods, a model-based correction method [16] was selected a priori for the primary comparison of the effect of low and high doses of atomoxetine on the corrected QT interval (QTcM) with that of placebo at 1, 2, 4, 6 and 12 h, and based on ECGs associated with individual subjects’ atomoxetine Cmax after dosing on day 7. As applied, this method provided a corrected value for the baseline subtracted least-squares (LS) mean difference between two treatments being compared and did not provide individual corrected QT values. QTc analyses were also performed using Fridericia's QT correction method (QTcF) [17] and the individual correction method (QTcI) developed by Malik [18, 19], but these corrections and analyses were considered secondary. In order to compute the individual correction factors, all individual ECGs (nine replicates per time point) from all time points (5 per day) for all off-treatment days (9 total, 4 pairs of lead-in days plus 1 day of placebo), 405 ECGs in total, were used. All three correction methods were also employed in the comparison between moxifloxacin and placebo.
Pharmacokinetic assessment
Blood samples were collected 15 min prior to and 1, 2, 4, 6 and 12 h after the morning dose on day 7 for atomoxetine and placebo treatments and on day 1 for moxifloxacin to measure plasma concentrations of atomoxetine and moxifloxacin. Blood samples were collected after obtaining ECGs. Samples obtained during the placebo period were not analyzed. Concentrations of atomoxetine were determined using a validated liquid chromatography-electrospray ionization-tandem mass spectrometry method [20]. The lower limit of quantification of the assay was 2.5 ng ml. Concentrations of moxifloxacin were determined using a validated liquid chromatography-turbo ion spray-tandem mass spectrometry method. The lower limit of quantification of the assay was 25 ng ml. Atomoxetine and moxifloxacin pharmacokinetic parameters were calculated using standard non-compartmental methods. WinNonlin Professional Edition version 5.2.1 (Pharsight Corp., Mountain View, CA, USA) was used to perform the analysis.
Sample size
The sample size was based on an intrasubject standard deviation of 9.433 ms with nine replicate ECG measurements and a 2 day baseline ECG assessment by using the RR covariate method [16]. One hundred and sixteen subjects provided over 99% power to conclude that the upper bound of two-sided 90% CI (equivalent to the upper bound of the one-sided 95% CI) was <10 ms, assuming the true mean difference between atomoxetine and placebo to be as much as 5 ms.
Statistical methods
The arithmetic means of these averaged QT and RR values at each time point on the 2 baseline days of each period were considered the baseline values for the corresponding time-matched post-dose measurements on day 7. Changes from baseline were calculated by subtracting baseline values from time-matched post-baseline values. Differences between treatments with respect to change from baseline were analyzed at each specific time point.
In addition to the analyses at the specific time points, mean QTc changes from baseline at each individual subject's Cmax on day 7 were compared between atomoxetine and placebo for both atomoxetine doses. The time point corresponding to the maximum concentrations of atomoxetine (tmax) on day 7 was determined for each subject for each dose level of atomoxetine. The QTc change from baseline at tmax for each atomoxetine dosing level and corresponding placebo dosing period were analyzed.
For the analysis of between-treatment differences in change in QTc, using QTcM, a mixed-effects analysis of covariance model was used with the RR interval change from baseline as a covariate, treatment, time and time-by-treatment as fixed effects, and subject, subject-by-time and subject-by-treatment as random effects. For the analysis of between-treatment differences in change in QTc, using QTcF and QTcI, a mixed-effect analysis of variance model was used with treatment, time and time-by-treatment as fixed effects, and subject, subject-by-time and subject-by-treatment as random effects. The identical models for the specific corrections were used for the moxifloxacin vs. placebo comparison.
The time-matched mean differences and the corresponding two-sided 90% CIs were computed. Atomoxetine was declared not different from placebo at a dose level when the upper limit of the two-sided 90% CI (equivalent to the upper limit of the one-sided 95% CI) for the largest time-matched mean difference between atomoxetine and placebo fell below 10 ms, assuming a constant variance at each time point.
Post hoc analyses were conducted to evaluate the potential for differences between Caucasians and Africans (ethnicity) with respect to between treatment differences in change in QTc, using all three heart rate correction methods. Ethnicity, ethnicity-by-treatment and ethnicity-by-time-by-treatment were added as fixed effects in the mixed effects analyses of variance and covariance models used for the a priori analyses.
Post hoc analyses were conducted to evaluate the potential for differences between Caucasians and Africans (ethnicity) with respect to the pharmacokinetic parameter of Cmax for both atomoxetine and moxifloxacin. A mixed-effect analysis of variance model was used with treatment (atomoxetine 20 mg twice daily, atomoxetine 60 mg twice daily, moxifloxacin), ethnicity, and ethnicity-by-treatment as fixed effects, and subject as a random effect.
Concentration–effect analyses
The relationships between placebo-subtracted changes from baseline in QTc (ΔΔQTc), I and F, and plasma concentrations of atomoxetine and moxifloxacin were analyzed through linear mixed effects modelling. In accordance with methods described in the literature [21], a mixed effects analysis of variance model was run with placebo-subtracted changes from baseline as the dependent variable and plasma concentrations of atomoxetine and moxifloxacin as the independent variable. The model included treatment as a fixed effect and subject as a random effect. Slope estimates and their 90% CI were generated for atomoxetine and moxifloxacin. QTcM values resulting from the a priori primary correction and analytical model for this study were not plotted against concentration data because, as applied, the model did not produce individual QTc values for each ECG or ECG replicate set. The model only produced estimates of LS means to compare between treatments, effectively resulting only in a corrected difference in QTc between treatments.
Assay sensitivity
Moxifloxacin was used not as a comparator but as a positive control expected to increase the QTc interval to establish assay sensitivity. Moxifloxacin's effect on QTcM (QTcI and QTcF were also assessed) was compared with that of placebo at 2, 4 and 6 h after dosing (day 1 of moxifloxacin and day 7 of placebo) using the same methods and analysis models described above. Although ECGs were collected at 1 and 12 h following moxifloxacin administration, the 2, 4 and 6 h post-moxifloxacin administration ECGs were prospectively declared as the moxifloxacin ECGs that would be used for comparison with placebo. These analyses were conducted with only moxifloxacin and placebo in the model. Assay sensitivity was assessed by two alternative tests. The first test (a priori, primary test for assay sensitivity per protocol Statistical Analysis Plan) determined whether the lower bound of the two-sided 95% CI around the maximum, time-matched, baseline-adjusted mean difference between moxifloxacin and placebo, close to 5 ms, was greater than zero. This test was consistent with the ICH E14 description of the purpose of the positive control that was in place at the time the protocol was developed between April 30 2007 and August 3 2007, which stated, ‘The confidence of the ability of the study to detect QT/QTc prolongation can be greatly enhanced by the use of a concurrent positive control group (pharmacological or non-pharmacological) to establish assay sensitivity. The positive control should have an effect on the mean QT/QTc interval of about 5 ms (i.e. an effect that is close to the QT/QTc effect that represents the threshold of regulatory concern, around 5 ms). Detecting the positive control's effect will establish the ability of the study to detect such an effect of the study drug.’ 1 The purpose of this a priori test was to establish whether the study had sufficient power to detect a statistically significant difference (in a conventional test of significance, e.g. two-sided test with alpha of 0.05 and a null hypothesis of equivalence) between moxifloxacin and placebo when the mean difference in effect between the treatments was about 5 ms.
The second test (post hoc), published by Zhang in 2008 [22] subsequent to the development of the protocol for this study, has become the test preferred by many regulatory authorities. Rather than establishing that the study has sufficient power to detect a 5 ms change, the purpose of this test is to establish that the positive control produces a magnitude of effect, relative to placebo, that is generally expected based on historical control. This post hoc test determined whether the lower bound of the one-sided 95% CI around the maximum, time-matched, baseline-adjusted mean difference between moxifloxacin and placebo exceeded 5 ms. A resampling-based multiple test [23] was carried out to adjust for multiplicity for both assay sensitivity tests.
Post hoc assessment of QT correction methods
In order to assess the quality of correction methods for the atomoxetine data and compare them, the linear mixed effects model regression slope of QTcF and QTcI vs. RR for each individual subject on atomoxetine treatment was computed. The average sum of squares of these individual slopes were then compared between correction methods [24]. The model-based, a priori, primary correction method for this study could not be compared with the QTcF and QTcI correction methods because the model does not produce individual QTc values for each ECG or ECG replicate set, as previously described.
Results
Subject disposition and demographics
Of the 10 027 men screened for PM status, 285 were identified as PMs and underwent further protocol screening, 131 were enrolled and received at least 1 dose of study treatment, nine were discontinued from the study and 122 completed the study. Of the subjects who discontinued, five withdrew as a result of an adverse event, two were lost to follow-up, one had a conflict with the study schedule and one had a positive urine drug screen. Twenty-five subjects were Caucasian, and 106 subjects were African. Other demographic characteristics were comparable among the four sequence groups (Table 1).
Table 1.
Demographic characteristics of the study subjects (n = 131)
| Characteristic | Group 1 (n = 33) | Group 2 (n = 33) | Group 3 (n = 32) | Group 4 (n = 33) |
|---|---|---|---|---|
| Origin, n (%) | ||||
| Caucasian | 6 (18.2) | 6 (18.2) | 5 (15.6) | 8 (24.2) |
| African | 27 (81.8) | 27 (81.8) | 27 (84.4) | 25 75.8) |
| Age (years) | 30.2 ± 10.89 | 31.1 ± 11.12 | 28.4 ± 9.80 | 30.1 ± 11.06 |
| BMI (kg m–2) | 22.33 ± 3.727 | 22.01 ± 3.237 | 22.61 ± 3.310 | 22.71 ± 3.180 |
| Weight (kg) | 67.29 ± 11.712 | 66.08 ± 11.524 | 67.59 ± 11.528 | 68.83 ± 11.250 |
| Height (cm) | 173.57 ± 6.329 | 173.02 ± 6.479 | 172.69 ± 6.960 | 173.91 ± 5.891 |
Age, BMI, weight and height are listed as mean ± SD. BMI, body mass index.
Pharmacokinetic evaluation
Geometric means of maximum steady-state plasma atomoxetine concentrations were 827 ng ml and 2770 ng ml observed at a median of 2 h post-dose for the 20 mg twice daily and 60 mg twice daily doses, respectively. Maximum steady-state plasma atomoxetine concentrations of 1711 ng ml and 5016 ng ml were observed for the 20 mg twice daily and 60 mg twice daily doses, respectively. The geometric mean of the maximum plasma moxifloxacin concentrations was 1780 ng ml observed at a median of 2 h post-dose. A maximum steady-state plasma moxifloxacin concentration of 3692 ng ml was observed.
QTc assessments
Table 2 shows statistical comparisons of the LS mean change from baseline in QTc between atomoxetine and placebo. The maximum LS mean difference in QTcM between atomoxetine 60 mg twice daily and placebo was 4.2 (two-sided 90% CI [upper bound of the two-sided 90% CI equivalent to the upper bound of the one-sided 95% CI] 2.5, 6.0) ms at 2 h post-dose. Regardless of the correction method, the upper limit of the two-sided 90% CI (equivalent to the upper bound of the one-sided 95% CI) for the mean difference between atomoxetine and placebo was <10 ms at all time points. The QTc effects for atomoxetine at Cmax for each individual subject were consistent with effects seen at the specific time points. The time point of ECG used in this analysis could vary for each individual subject and was determined by the time point associated with the peak plasma concentration for the individual subject.
Table 2.
Statistical comparison of least-squares mean ΔQTc between atomoxetine and placebo
| Parameter (ms) | Time (h) | Least-squares mean ΔQTc | Least-squares mean difference (two-sided 90% CI) Atomoxetine 20 mg twice daily−placebo | Least-squares mean difference (two-sided 90% CI) Atomoxetine 60 mg twice daily−placebo | ||
|---|---|---|---|---|---|---|
| Placebo (n = 126) | Atomoxetine 20 mg twice daily (n = 126) | Atomoxetine 60 mg twice daily (n = 125) | ||||
| Model-based QTc | 1 | −3.2 | −3.2 | −0.9 | -0.0 (−1.7, 1.7) | 2.3 (0.6, 4.0) |
| 2 | −1.9 | −1.4 | 2.3 | 0.5 (−1.2, 2.2) | 4.2 (2.5, 6.0) | |
| 4 | −0.8 | −2.2 | 3.1 | −1.5 (−3.2, 0.2) | 3.8 (2.1, 5.6) | |
| 6 | −1.6 | −3.6 | −0.2 | −2.0 (−3.7, −0.3) | 1.4 (−0.3, 3.1) | |
| 12 | −4.3 | −5.4 | −2.4 | −1.1 (−2.7, 0.6) | 1.9 (0.2, 3.6) | |
| Cmax | −2.7 | −3.5 | −0.02 | −0.8 (−2.6, 1.1) | 2.7 (0.7, 4.7) | |
| QTcF | 1 | −3.8 | −3.9 | −1.1 | −0.1 (−1.7, 1.5) | 2.7 (1.1, 4.3) |
| 2 | −2.7 | −2.4 | 1.9 | 0.3 (−1.3, 1.9) | 4.6 (3.0, 6.2) | |
| 4 | −1.3 | −3.0 | 3.1 | −1.7 (−3.3, −0.1) | 4.4 (2.8, 6.0) | |
| 6 | −1.8 | −3.7 | 0.4 | −1.9 (−3.5, −0.3) | 2.2 (0.6, 3.8) | |
| 12 | −4.6 | −5.7 | 2.0 | −1.0 (−2.6, 0.6) | 2.6 (1.0, 4.2) | |
| Cmax | −2.9 | −2.8 | 1.4 | 0.1 (−1.4, 1.6) | 4.4 (2.9, 5.9) | |
| QTcI | 1 | −4.4 | −6.0 | −3.9 | −1.6 (−3.3, 0.1) | 0.6 (−1.1, 2.3) |
| 2 | −3.4 | −4.3 | −1.0 | −0.9 (−2.6, 0.9) | 2.4 (0.7, 4.1) | |
| 4 | −1.9 | −5.1 | −0.2 | −3.2 (−4.9, −1.5) | 1.7 (−0.0, 3.4) | |
| 6 | −2.4 | −5.3 | −2.4 | −2.9 (−4.6, −1.2) | 0.0 (−1.7, 1.7) | |
| 12 | −5.3 | −7.5 | −4.7 | −2.2 (−3.9, −0.5) | 0.7 (−1.1, 2.4) | |
| Cmax | −3.8 | −4.8 | −1.3 | −1.0 (−2.6, 0.5) | 2.4 (0.9, 4.0) | |
Cmax, maximum atomoxetine concentration; QTcF, Fridericia QT correction; QTcI, individual QT correction; ΔQTc, change in QTc from baseline.
From the QTc results, no subject had QTcF or QTcI >500 ms during the study. One subject had a QTcF interval >480 ms following moxifloxacin. No subject, treated with any dose, at any time point had an increase from baseline in QTcF and QTcI >60 ms. Three subjects (atomoxetine 60 mg twice daily group) had an increase from baseline in QTcF and QTcI >30 ms at multiple time points.
Potential influence of ethnicity on pharmacokinetics of atomoxetine and moxifloxacin and changes in QTc
Differences in changes between atomoxetine and placebo for QTc were numerically greater among Caucasians subjects than African subjects, but these differences were not statistically significant. The P values for ethnicity and ethnicity-by-treatment for all corrections were greater than 0.1000. The analyses failed to support a difference between ethnic groups with respect to change in QTc and failed to support a difference between ethnic groups with respect to the influence of atomoxetine on change in QTc.
Differences in QTc changes between moxifloxacin and placebo were numerically less among Caucasian subjects than African subjects. For moxifloxacin compared with placebo, the P values for ethnicity were: QTcM 0.0508, QTcF 0.0438, QTcI 0.0830, suggesting a statistical difference in change in QTc between ethnic groups. However, the P values for ethnicity-by-treatment were all greater than 0.4000, failing to support a statistical difference between ethnic groups with respect to the influence of moxifloxacin on change in QTc.
For both atomoxetine 20 mg twice daily and 60 mg twice daily, and for moxifloxacin, mean Cmax among Caucasians was numerically lower than among Africans (atomoxetine 20 mg twice daily, Caucasians: 818.4 ± 242.1 μg ml, Africans: 874.9 ± 250.0 μg ml; atomoxetine 60 mg twice daily Caucasians: 2658.6 ± 671.2 μg ml, Africans: 2950.1 ± 855.4 μg ml; moxifloxacin −1628.8 ± 279.5 μg ml for Caucasians and 1888.9 ± 506.0 μg ml for Africans). However, the P value for treatment-by-ethnicity was not significant (P = 0.3165), failing to support a statistical difference between ethnic groups.
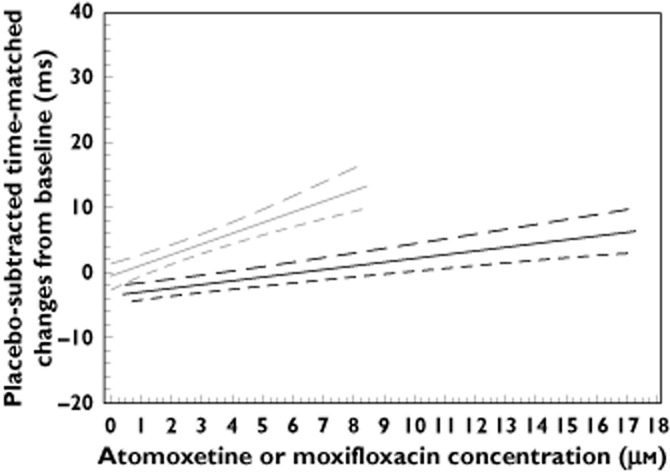
Concentration–effect analysis: relationships between placebo-subtracted change from baseline (ΔΔQTc) and plasma atomoxetine and moxifloxacin concentrations
For illustrative purposes, Figure 2 shows the relationship between ΔΔQTcI and plasma concentrations of atomoxetine and moxifloxacin. The slope estimates for both atomoxetine and moxifloxacin were significantly different from zero for both QTcI (P < 0.0001) and QTcF (P < 0.0001). Expressed in units of ms/(ng ml), the following slopes were observed for moxifloxacin and atomoxetine, respectively: 0.00382 and 0.00191 for QTcI, as well as 0.00395 and 0.00252 for QTcF.
Figure 2.
Plot of ΔΔQTcI vs. plasma atomoxetine and moxifloxacin concentrations following 7 days of atomoxetine (20 mg twice daily or 60 mg twice daily) and a single 400 mg dose of moxifloxacin.  , Atomoxetine least-squares mean;
, Atomoxetine least-squares mean;  , Moxifloxacin least-squares mean;
, Moxifloxacin least-squares mean;  , atomoxetine 90% lower bound;
, atomoxetine 90% lower bound;  , moxifloxacin 90% lower bound;
, moxifloxacin 90% lower bound;  , atomoxetine 90% upper bound;
, atomoxetine 90% upper bound;  , moxifloxacin 90% upper bound
, moxifloxacin 90% upper bound
In Figure 2, the lower bounds of the two-sided 90% CI (equivalent to the lower bound of the one-sided 95% CI) for ΔΔQTcI and plasma concentration of moxifloxacin reached 5 ms at a concentration of 5.9 μm (equivalent to 2588 ng ml), that was 70.1% of the maximum observed concentration of moxifloxacin (3692 ng ml) and the regression line exceeded 10 ms within the Cmax concentrations observed in the study. The upper bound of the two-sided 90% CI (equivalent to the upper bound of the one-sided 95% CI) for ΔΔQTcI and atomoxetine plasma concentration reached 6.72 ms (<10 ms) at the maximum concentration of 17.2 μm (equivalent to 5016 ng ml) observed with atomoxetine.
Assay sensitivity
Table 3 shows the two statistical comparisons between moxifloxacin and placebo. By all correction methods at all time points moxifloxacin resulted in statistically significantly larger changes in QTc than did placebo in a two-sided test at an α of 0.05. The differences in the mean change from baseline in QTcM for moxifloxacin vs. placebo ranged from 4.3 to 4.8 ms, with the lower bound of the two-sided 95% CI ranging from 2.5 to 3.0 ms. Moxifloxacin did not cause a 5 ms or greater increase in QTc compared with placebo by any of the correction methods at any time point. The lower bound of the two-sided 90% CI (equivalent to the lower bound of the one-sided 95% CI) ranged from 2.8 to 3.3 ms. At 12 h following administration, moxifloxacin still demonstrated a 4 ms mean increase in QTc from baseline, indicating that the effect of moxifloxacin on QTc did not decline in relation to the decline in moxifloxacin plasma concentrations.
Table 3.
Statistical comparison of least-squares mean ΔQTc between moxifloxacin 400 mg and placebo
| Parameter (msec) | Time (h) | Least-squares mean ΔQTc | Least-squares mean difference (two-sided 95% CI) Moxifloxacin−placebo | Least-squares mean difference (two-sided 90% CI) Moxifloxacin−placebo | |
|---|---|---|---|---|---|
| Placebo (n = 126) | Moxifloxacin 400 mg (n = 125) | ||||
| Model-based QTc | 2 | −2.3 | 2.0 | 4.3 (2.5, 6.1) | 4.3 (2.8, 5.8) |
| 4 | −1.1 | 3.8 | 4.8 (3.0, 6.7) | 4.8 (3.3, 6.4) | |
| 6 | −1.9 | 2.7 | 4.5 (2.7, 6.4) | 4.5 (3.0, 6.1) | |
| QTcF | 2 | −2.7 | 1.9 | 4.7 (2.8, 6.5) | 4.7 (3.1, 6.2) |
| 4 | −1.3 | 3.7 | 5.0 (3.2, 6.8) | 5.0 (3.5, 6.5) | |
| 6 | −1.8 | 2.7 | 4.6 (2.7, 6.4) | 4.6 (3.0, 6.1) | |
| QTcI | 2 | −3.4 | 1.4 | 4.9 (3.0, 6.7) | 4.9 (3.3, 6.4) |
| 4 | −2.0 | 3.0 | 5.0 (3.1, 6.8) | 5.0 (3.4, 6.5) | |
| 6 | −2.4 | 2.3 | 4.7 (2.9, 6.6) | 4.7 (3.2, 6.3) | |
QTcF, Fridericia QT correction; QTcI, individual QT correction; ΔQTc, change in QTc from baseline.
Post hoc comparison of correction methods
For atomoxetine, the average sum of squared individual QTc−RR slopes for the linear regression lines were 0.0027 for the Fridericia correction method and 0.0022 for the individual correction method. As the individual correction method resulted in a slightly lower average sum of squared individual QTc−RR slopes for the linear regression lines it could be considered the slightly superior correction method.
Safety
No deaths occurred during the study. Three subjects experienced four serious adverse events (migraine, gastritis, focal epilepsy, malignant brain tumour) considered unrelated to study drug by the investigator. The focal epilepsy and malignant brain tumour were observed in the same subject.
One subject discontinued from the study during atomoxetine 20 mg twice daily treatment due to palpitations, dizziness and increased systolic blood pressure considered related to study drug by the investigator. Two subjects discontinued due to erectile dysfunction during atomoxetine 20 and 60 mg twice daily treatment, respectively, considered related to study drug by the investigator. Two subjects discontinued as a result of panic attacks and malignant brain tumour, respectively, considered unrelated to study drug by the investigator. The brain tumour leading to discontinuation is the same event as the brain tumour considered a serious adverse event noted above.
In total, there were 618 adverse events that were either new or worsened from prior to the first treatment period reported following atomoxetine administration; 400 were considered related to study drug by the investigator. Table 4 lists adverse events that were reported with an incidence of ≥5% during atomoxetine treatment and the incidence during atomoxetine treatment was greater than the incidence during placebo treatment. These events could be expected based on atomoxetine product labelling, except for application site reaction and nasopharyngitis. Application site reaction was reaction to the ECG electrodes applied to the skin and nasopharyngitis was likely intercurrent and not drug-related. The incidence of testicular pain and potentially similar events of suprapubic pain, bladder discomfort, and groin pain, all reported with an incidence of <5%, was higher than might be expected based on previous studies reflected in the product labelling of adverse events.
Table 4.
Percentages of subjects with adverse events that were new or worsened since study entry (percentage ≥5% and percentage with atomoxetine ≥ percentage with placebo)
| Adverse event | Placebo % | Moxifloxacin % | Atomoxetine % |
|---|---|---|---|
| Application site reaction | 48.1 | 19.1 | 51.1 |
| Postural orthostatic tachycardia syndrome | 3.8 | 3.1 | 23.7 |
| Testicular pain | 3.1 | 0.8 | 13.0 |
| Blood pressure increased | 9.2 | 3.8 | 12.2 |
| Nausea | 2.3 | 1.5 | 10.7 |
| Erectile dysfunction | 2.3 | 0.8 | 10.7 |
| Urinary hesitation | 1.5 | 0.0 | 10.7 |
| Dyspepsia | 3.1 | 1.5 | 9.9 |
| Dry mouth | 2.3 | 0.0 | 9.9 |
| Anorexia | 0.0 | 0.0 | 9.1 |
| Dizziness | 1.5 | 1.5 | 6.1 |
| Nasopharyngitis | 4.6 | 0.8 | 6.1 |
| Insomnia | 2.3 | 0.0 | 6.1 |
| Dysuria | 1.5 | 0.0 | 5.3 |
| Sinus tachycardia | 0.0 | 0.0 | 5.3 |
Both atomoxetine doses increased supine diastolic and systolic blood pressure and heart rate relative to placebo. Little apparent difference between doses was detected with respect to blood pressure, but there appeared to be a larger increase in heart rate with the higher dose. Atomoxetine was also associated with a significant orthostatic drop in blood pressure and a significant orthostatic rise in heart rate relative to placebo at all post-dose time points. However, the absolute orthostatic rise in heart rate with atomoxetine at either dose was actually modest, with the greatest observed value being a rise of 12.9 beats min at 1 h post-dose for the 60 mg twice daily dose. Clinical laboratory data (complete blood count, complete metabolic panel, urinalysis, hormonal, cholesterol and inflammation) revealed no clinically relevant findings.
Discussion
This report describes a TQT study that evaluated the effect of atomoxetine on cardiac repolarization at the maximum approved dose in a population of exclusively CYP2D6 PM subjects. Based on the a priori definition of noninferiority, both the low and high doses of atomoxetine were noninferior to placebo in the maximum mean difference in change from baseline in QTcM. Based on the a priori definition of assay sensitivity (sufficient statistical power to detect a 5 ms difference in effect between moxifloxacin and placebo) the study demonstrated assay sensitivity. However, based on the post hoc definition of assay sensitivity (moxifloxacin produces an effect comparable with that expected based on historical control), assay sensitivity was not demonstrated.
Secondary results using QTcI and QTcF correction methods support the interpretation of noninferiority for both atomoxetine doses compared with placebo. In an analysis that used the QTc difference between atomoxetine and placebo based on the time point associated with the Cmax observed for atomoxetine for each individual subject, the resulting upper bounds for the CIs were as follows: QTcM/20 mg twice daily = 1.1 ms; QTcM/60 mg twice daily = 4.7 ms.
However, three observations in this study might suggest an association between atomoxetine and QTc lengthening:
the maximum mean atomoxetine − placebo difference in QTcM with the 60 mg twice daily dose was similar to the difference between moxifloxacin and placebo;
the maximum mean atomoxetine 60 mg twice daily − placebo difference was higher than the difference between atomoxetine 20 mg twice daily and placebo, suggesting a positive dose–response relationship;
slopes of the linear regression lines fited to the relationship between plasma concentration for atomoxetine and the atomoxetine − placebo difference in QTcI and QTcF were positive and statistically significantly different from zero.
The matters of the similarity between the effect of the atomoxetine 60 mg twice daily dose and moxifloxacin as well as lack of confirmation of assay sensitivity based on the Zhang definition [22] must be considered in the context of the pharmacokinetics of moxifloxacin observed in this study.
Moxifloxacin Cmax concentrations in this study were lower than those reported in the literature. Bloomfield and colleagues [25] described the pharmacokinetic characteristics of a 400 mg oral moxifloxacin dose administered to 20 subjects along with its expected effect on QTc: Cmax = 2236.8 (95% CI 2001.4, 2499.9) ng ml. In the present study, the moxifloxacin geometric mean Cmax was 1780 (95% CI 1708, 1861) ng ml, which is lower than other reported values in the literature (79.6% of the value reported by Bloomfield and colleagues). A reduction in Cmax for moxifloxacin could contribute to a reduction in the QTc increase relative to placebo based on historical experience, and this would result in a less than expected difference between moxifloxacin and atomoxetine as well as an inability to establish assay sensitivity based on the Zhang definition [22]. The underlying reasons for these lower moxifloxacin concentrations are unknown, but could reflect either poor absorption or potential differences in intrinsic factors that influence pharmacokinetics of oral moxifloxacin. Pertinent to the design characteristics of the present study, an analysis by the United States Food and Drug Administration (FDA) of pooled moxifloxacin data from 20 TQT studies showed that: (i) food (fed within 3 h of moxifloxacin administration) decreased moxifloxacin's absorption rate constant by 27% compared with moxifloxacin taken in a fasted state, with higher Cmax in fasted vs. fed subjects significant at P < 0.01 and (ii) higher moxifloxacin exposures observed in women compared with men are explained by women's lower body weight [26]. The FDA authors [26] note, based on the research of others, that total exposure to moxifloxacin (area under the concentration–time curve) would not change substantially with administration in a fed state but that time of maximum concentration would shift toward a later time subsequent to administration. In the present study, subjects received their moxifloxacin 2 h after a meal rather than after an overnight fast, and all subjects were men.
The pharmacokinetic–pharmacodynamic analysis for moxifloxacin could be considered to support assay sensitivity for this study. The slope of the linear regression line for moxifloxacin plasma concentration, ΔΔQTcF, 0.00395 ms/(ng ml), was within the historically expected range. Bloomfield and colleagues [26] reported a linear regression line slope, for QTcF of 0.0039 ms/(ng ml). Based on the FDA analysis of 20 TQT studies noted above, Florian and colleagues [26] have reported a mean regression line slope for QTcF of 0.00306 ms/(ng ml), with a range of 0.0016–0.0048 ms/(ng ml) and a 90% CI of 0.00281, 0.00330 ms/(ng ml).
Regarding whether atomoxetine influences QTc, a positive and significant concentration−response relationship supports the proposition that atomoxetine does increase QTc to some extent. However, the pharmacokinetic−pharmacodynamic analysis found that for atomoxetine, the upper bound of the one-sided 95% CI for ΔΔQTcI regression line did not reach 10 ms at the highest atomoxetine concentration observed, supporting the proposition that atomoxetine did not have a clinically concerning influence on QTc in this study. Therefore, the magnitude of influence, based on the concentration−response relationship observed appears modest for the doses administered in these subjects.
Although no statistically significant difference was found between African and Caucasian subjects with respect to the effect of atomoxetine or moxifloxacin on QTc, it cannot be concluded that this was not a type II error in light of numerical differences. Likewise, while the differences in atomoxetine and moxifloxacin Cmax values between African and Caucasian subjects were not statistically significant, it cannot be concluded that these were not type II errors. These ethnic findings should be interpreted with extreme caution because this study was not designed to evaluate the potential influence of ethnicity on pharmacokinetics or changes in QTc. If these had been primary or secondary aims of the study, then sample sizes would have been equal, and more extensive pharmacokinetic data would have been collected. It is impossible to know the true impact, if any, of the uneven ethnic distribution of subjects.
Exclusively male subjects were used in this study in order to reduce variance in QTc changes that can arise when using premenopausal women who demonstrate cyclical changes in baseline QTc and lengthening in QTc in response to drugs that prolong ventricular repolarization related to endocrine changes with the menstrual cycle [27–29]. This could, however, represent a limitation of the study, because women can show greater change in QTc when exposed to drugs delaying ventricular repolarization [30]. This potential limitation was balanced by the use of exclusively CYP2D6 PM subjects and the other steps taken to maximize exposure to atomoxetine.
Acknowledgments
The authors thank the investigators and staff from Parexel International (South Africa) (Pty.) Ltd who conducted this clinical study and the subjects who participated. This study was funded by Eli Lilly and Company (Indianapolis, IN, USA).
Competing Interests
H. Haber is an employee of i3Statprobe, Inc. P. Kothare is an employee of Merck & Co., Inc. L. Kauffman is an employee of Parexel International. All authors apart from H. Haber are shareholders of Eli Lilly and Company. All authors apart from H. Haber, P. Kothare and L. Kauffman are employees of Eli Lilly and Company.
References
- 1.ICH. Guidance for industry E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Available at http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm129357.pdf (last accessed 1 August 2012)
- 2.Scherer D, Hassel D, Bloehs R, Zitron E, von Löwenstern K, Seyler C, Thomas D, Konrad F, Bürgers HF, Seemann G, Rottbauer W, Katus HA, Karle CA, Scholz EP. Selective noradrenaline reuptake inhibitor atomoxetine directly blocks hERG currents. Br J Pharmacol. 2009;156:226–236. doi: 10.1111/j.1476-5381.2008.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milnes JT, Witchel HJ, Leaney JL, Leishman DJ, Hancox JC. Investigating dynamic protocol-dependence of hERG potassium channel inhibition at 37 degrees C: cisapride versus dofetilide. J Pharmacol Toxicol Methods. 2010;61:178–191. doi: 10.1016/j.vascn.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Sauer JM, Ring BJ, Witcher JW. Clinical pharmacokinetics of atomoxetine. Clin Pharmacokinet. 2005;44:571–590. doi: 10.2165/00003088-200544060-00002. [DOI] [PubMed] [Google Scholar]
- 5.Simpson D, Plosker GL. Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs. 2004;64:205–222. doi: 10.2165/00003495-200464020-00005. [DOI] [PubMed] [Google Scholar]
- 6.Webster R, Leishman D, Walker D. Towards a drug concentration effect relationship for QT prolongation and torsades de pointes. Curr Opin Drug Discov Devel. 2002;5:116–126. [PubMed] [Google Scholar]
- 7.Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 8.Terrar DA, Wilson CM, Graham SG, Bryant SM, Heath BM. Comparison of guinea-pig ventricular myocytes and dog Purkinje fibers for in vitro assessment of drug-induced delayed repolarization. J Pharmacol Toxicol Methods. 2007;56:171–185. doi: 10.1016/j.vascn.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Wernicke JF, Faries D, Girod D, Brown J, Gao H, Kelsey D, Quintana H, Lipetz R, Michelson D, Heiligenstein J. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Saf. 2003;26:729–740. doi: 10.2165/00002018-200326100-00006. [DOI] [PubMed] [Google Scholar]
- 10.Sawant S, Daviss SR. Seizures and prolonged QTc with atomoxetine overdose. Am J Psychiatry. 2004;161:757. doi: 10.1176/appi.ajp.161.4.757. [DOI] [PubMed] [Google Scholar]
- 11.Barker MJ, Benitez JG, Ternullo S, Juhl GA. Acute oxcarbazepine and atomoxetine overdose with quetiapine. Vet Hum Toxicol. 2004;46:130–132. [PubMed] [Google Scholar]
- 12.Kashani J, Ruha AM. Isolated atomoxetine overdose resulting in seizure. J Emerg Med. 2007;32:175–178. doi: 10.1016/j.jemermed.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Williams EJ. Experimental designs balanced for the estimation of residual effects of treatments. Aust J Sci Res. 1949;2:149–168. [Google Scholar]
- 14.Beasley CM, Mitchell MI, Dmitrienko AA, Emmick JT, Shen W, Costigan TM, Bedding AW, Turik MA, Bakhtyari A, Warner MR, Ruskin JN, Cantilena LR, Jr, Kloner RA. The combined use of ibutilide as an active control with intensive electrocardiographic sampling and signal averaging as a sensitive method to assess the effects of tadalafil on the human QT interval. J Am Coll Cardiol. 2005;46:678–687. doi: 10.1016/j.jacc.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Couderc JP, Xiaojuan X, Zareba W, Moss AJ. Assessment of the stability of the individual-based correction of QT interval for heart rate. Ann Noninvasive Electrocardio. 2005;10:25–34. doi: 10.1111/j.1542-474X.2005.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dmitrienko A, Smith B. Repeated-measures models in the analysis of QT interval. Pharmaceut Statist. 2003;2:175–190. [Google Scholar]
- 17.Fridericia LS. Die Sytolendauer in Elektrokardiogramm bei normalen Menshen und bei Herzkranken. Acta Med Scand. 1920;53:469–486. [Google Scholar]
- 18.Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001;12:411–420. doi: 10.1046/j.1540-8167.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 19.Salvi V, Karnad DR, Panicker GK, Kothari S. Update on the evaluation of a new drug for effects on cardiac repolarization in humans: issues in early drug development. Br J Pharmacol. 2010;159:34–48. doi: 10.1111/j.1476-5381.2009.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullen JH, Shugert RL, Ponsler GD, Li Q, Sundaram B, Coales HL, Yakupkovic JE, Lelacheur RM, Wheeler WJ, Belas FJ, Sauer JM. Simultaneous quantification of atomoxetine as well as its primary oxidative and O-glucuronide metabolites in human plasma and urine using liquid chromatography tandem mass spectrometry (LC/MS/MS) J Pharm Biomed Anal. 2005;38:720–733. doi: 10.1016/j.jpba.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Garnett CE, Beasley N, Bhattaram VA, Jadhav PR, Madabushi R, Stockbridge N, Tornøe CW, Wang Y, Zhu H, Gobburu JV. Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol. 2008;48:13–18. doi: 10.1177/0091270007307881. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J. Testing for positive control activity in a thorough QTc study. J Biopharm Stat. 2008;18:517–528. doi: 10.1080/10543400801995478. [DOI] [PubMed] [Google Scholar]
- 23.Westfall PH, Young SS. Resampling-Based Multiple Testing: Examples and Methods for P-Value Adjustment. New York: Wiley; 1993. [Google Scholar]
- 24.Tornøe CW, Garnett CE, Wang Y, Florian J, Li M, Gobburu JV. Creation of a knowledgement management system for QT analyses. J Clin Pharmacol. 2011;51:1035–1042. doi: 10.1177/0091270010378408. [DOI] [PubMed] [Google Scholar]
- 25.Bloomfield DM, Kost JT, Ghosh K, Hreniuk D, Hickey LA, Guitierrez MJ, Gottesdiener K, Wagner JA. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin Pharmacol Ther. 2008;84:475–480. doi: 10.1038/clpt.2008.33. [DOI] [PubMed] [Google Scholar]
- 26.Florian JA, Tornøe CW, Brundage R, Parekh A, Garnett CE. Population pharmacokinetic and concentration-QTc models for moxifloxacin: pooled analysis of 20 thorough QT studies. J Clin Pharmacol. 2011;51:1152–1162. doi: 10.1177/0091270010381498. [DOI] [PubMed] [Google Scholar]
- 27.Burke JH, Ehlert FA, Kruse JT, Parker MA, Goldberger JJ, Kadish AH. Gender-specific differences in the QT interval and the effect of autonomic tone and menstrual cycle in healthy adults. Am J Cardiol. 1997;79:178–181. doi: 10.1016/s0002-9149(96)00707-2. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez I, Kilborn MJ, Liu X-K, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa M, Ooie T, Takahashi N, Taniguchi Y, Anan F, Yonemochi H, Saikawa T. Influence of menstrual cycle on QT interval dynamics. Pacing Clin Electrophysiol. 2006;29:607–613. doi: 10.1111/j.1540-8159.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 30.Somberg JC, Preston RA, Ranade V, Cvetanovic I, Molnar J. Gender differences in cardiac repolarization following intravenous sotalol administration. J Cardiovasc Pharmacol Ther. 2012;17:86–92. doi: 10.1177/1074248411406505. [DOI] [PubMed] [Google Scholar]


