Figure 2.
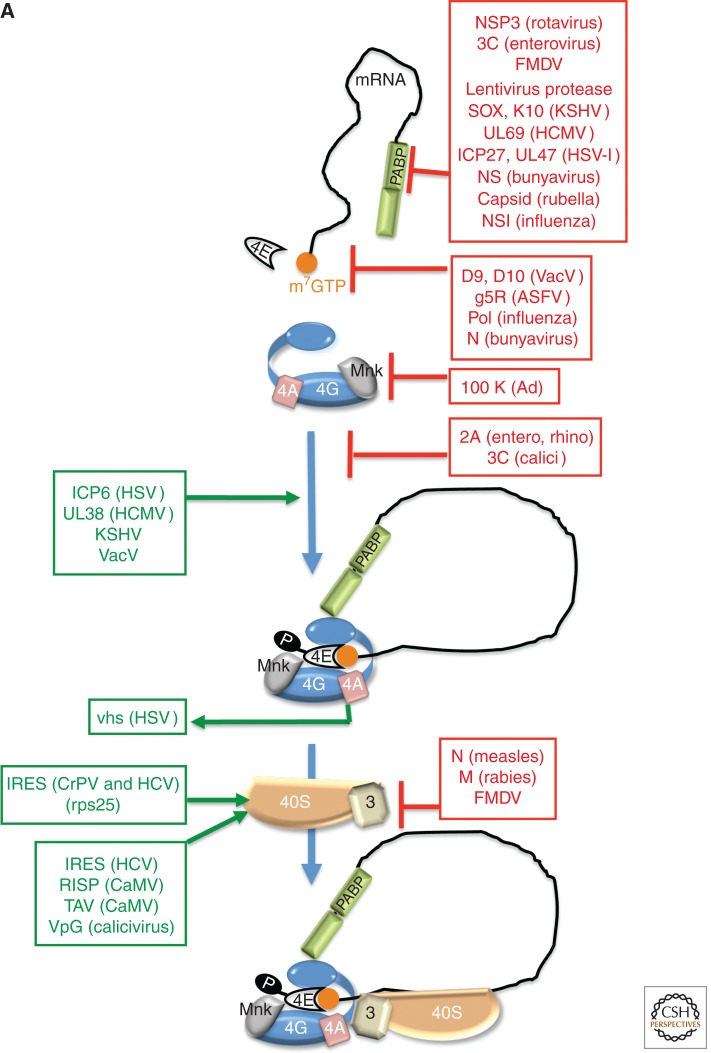
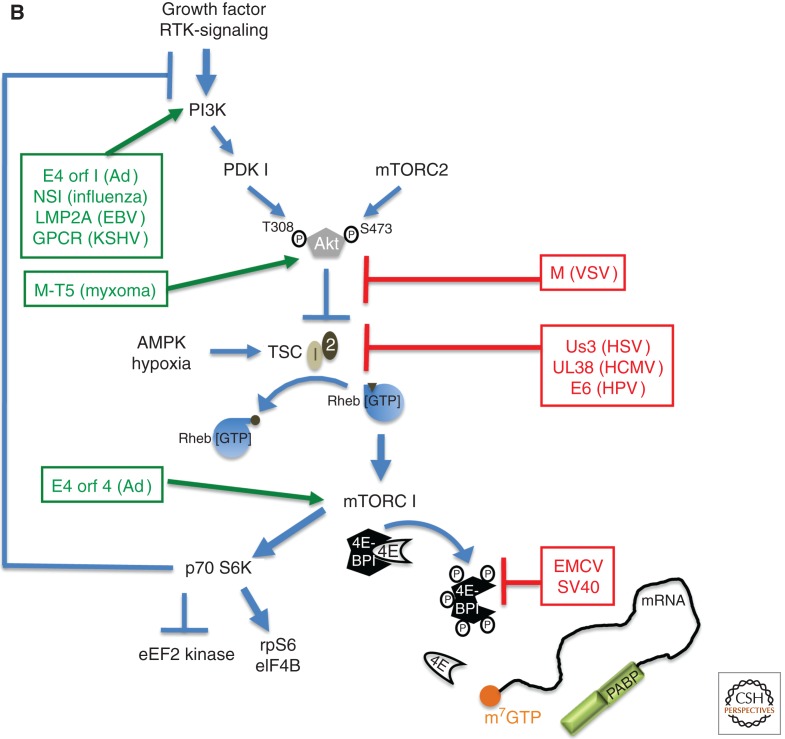
Control of translation by virus-encoded functions that regulate assembly of eIF4F. (A) eIF4E (4E) is able to interact with eIF4G and assemble the multi-subunit eIF4F complex (4E, 4A, 4G) on the m7GTP-capped (orange ball) mRNA 5′ end. eIF4F assembly typically results in eIF4E phosphorylation by the eIF4G-associated kinase Mnk and recruits eIF3 bound to the 40S ribosome subunit together with associated factors in the 43S complex. PABP is depicted bound to the 3′-poly(A) tail and associates with eIF4G to stimulate translation. Virus-encoded factors that activate (green, on the left) and repress (red, to the right) cellular functions are shown. (B) Cell signaling pathways that control the activity of the translational repressor 4E-BP1 and regulate eIF4F assembly allow for rapid changes in gene expression programs in response to a variety of physiological cues, including viral infection. In response to growth factor–stimulated receptor tyrosine kinases (RTK), PI-3-kinase (PI3K) and mTORC2 activate Akt by phosphorylation on T308 and S473, which, in turn, represses the tuberous sclerosis complex (TSC) by phosphorylating the TSC2 subunit. TSC is a GTPase-activating protein (GAP) that limits the activity of Rheb by promoting Rheb·GDP accumulation, which represses mTORC1. Whereas AMPK and hypoxia stimulate TSC GAP activity to inhibit mTORC1, Receptor Tyrosine Kinase (RTK)–mediated Akt activation inhibits TSC, resulting in mTORC1 activation. Inhibiting TSC allows rheb·GTP accumulation and mTORC1 activation, and results in p70S6K and 4E-BP1 phosphorylation. By stimulating ribosomal protein S6 phosphorylation (rpS6), p70 S6K activation by mTORC1 stimulates the eIF4A-accessory factor eIF4B and inhibits eukaryotic elongation factor 2 (eEF2) kinase, stimulating elongation. Normally, p70 S6K activation represses PI3-kinase (PI3K) to feedback and limit mTORC1 activation. 4E-BP1 hyperphosphorylation (depicted as circled P) relieves translational repression and releases eIF4E.