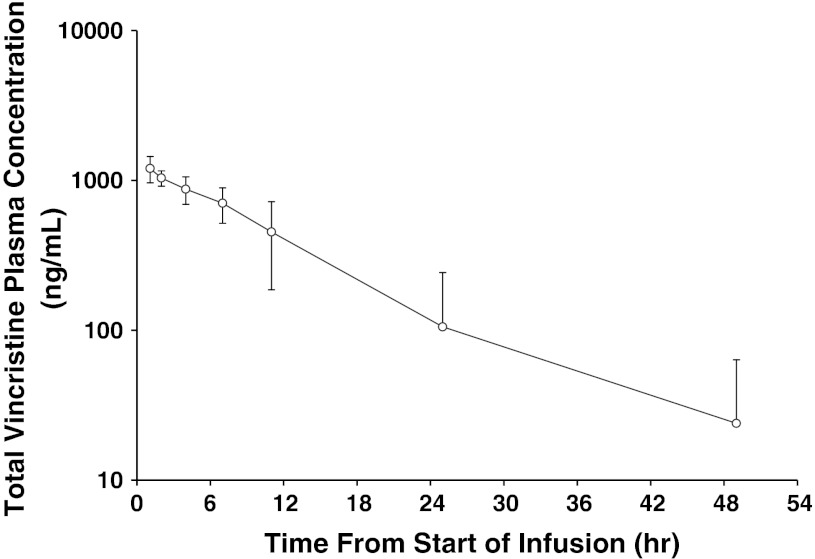
Fig. 5.
Mean plasma concentration–time profile of total VCR in humans following IV administration of VSLI at 2.25 mg/m2. Plasma was collected from adult Ph-chromosome-negative relapsed/refractory patients, N = 12. Total VCR concentrations were measured at the indicated times post-dose of VSLI using a validated LC/MS–MS method. The lower limit of quantitation (LLOQ) for vincristine sulfate was 1.00 ng/mL. Pharmacokinetic parameters for total VCR concentrations (encapsulated and free) in plasma were calculated from the plasma concentration–time data using a noncompartmental analysis (NCA) method (WinNonlin Professional Network Edition, Version 5.2, Pharsight Corp, Palo Alto, CA, USA)