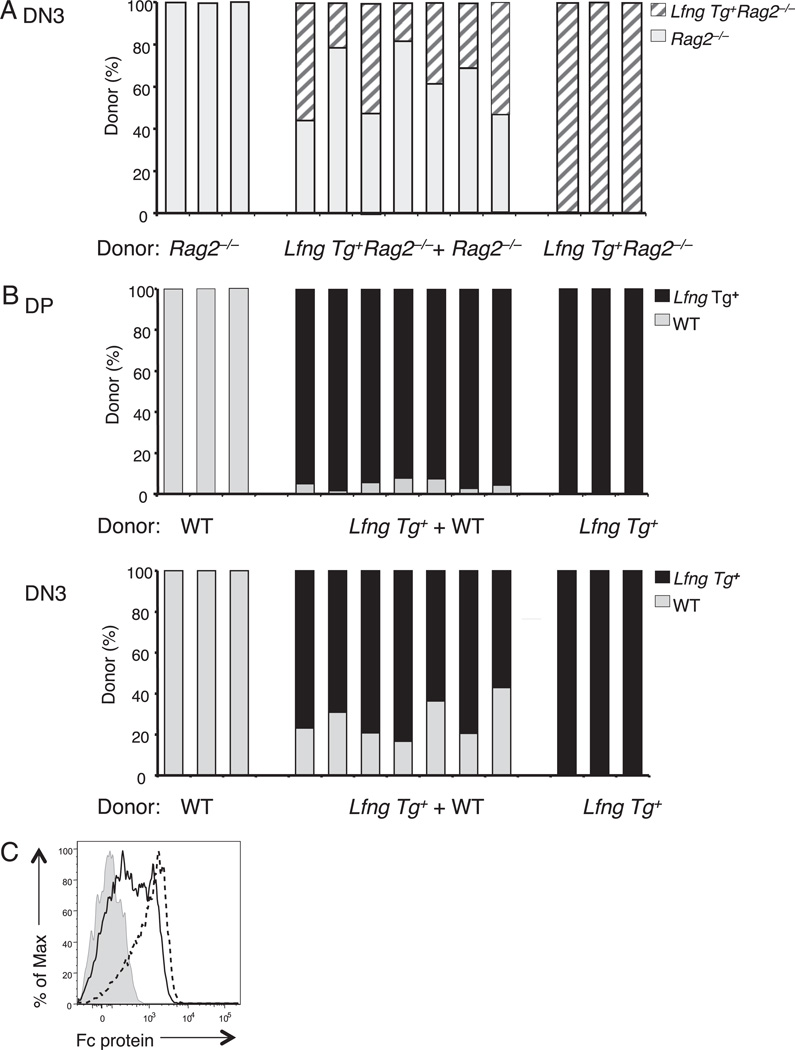
FIGURE 1.
The competitive advantage of the Lfng transgene depends on V(D)J recombination. A, Lin− bone marrow progenitors from Lfng Tg+ Rag2−/− (B6.CD45.2) and Rag2−/− (B6.CD45.1) donors were injected i.v. in equal ratios into lethally irradiated WT hosts (B6.CD45.1;CD45.2). The relative contribution of Lfng Tg+Rag2−/− and Rag2−/− donors to the DN3 thymocyte pool was assessed 3 wk later by staining total thymocytes with Abs against CD4, CD8, CD25, CD117, CD45.1, and CD45.2. B, Lin− bone marrow progenitors from Lfng Tg+ and WT donors were intrathymically injected separately or together as a 50:50 mix into sublethally irradiated WT hosts. The relative contribution of Lfng Tg+ and WT donors to the DP and DN3 thymocyte pools was assessed 3 wk later by staining total thymocytes with Abs against CD4, CD8, CD25, CD117, CD45.1, and CD45.2. C, Lfng overexpression enhances DL4-Fc binding by Rag2−/− DN3a thymocytes. Flow cytometry was used to measure binding of DL4-Fc protein by DN3a (CD117−CD25+) thymocytes from Lfng Tg+Rag2−/− (dashed line) compared with Rag2−/− (solid line) mice. Binding of control Fc protein to Lfng Tg+ Rag2−/− DN3a thymocytes is shown for comparison (shaded line). Binding of control Fc protein to Rag2−/− DN3a thymocytes had a pattern similar to Lfng Tg+Rag2−/− DN3 thymocytes (data not shown). Each experiment was performed at least three times with similar results.