Abstract
The optimal duration of dual antiplatelet therapy (DAPT) after drug-eluting stent (DES) implantation is an important, unanswered question. This study was designed to evaluate the association of varying durations of DAPT on clinical outcomes after DES implantation for the treatment of coronary artery disease. Using the National Heart, Lung and Blood Institute (NHLBI) Dynamic Registry, patients enrolled in the last two waves after index percutaneous coronary intervention (PCI) with DES and who were event free at time of landmark analysis were included. Landmark analysis was performed at 12 and 24 months after PCI and patients stratified according to continued use of DAPT or not. Subjects were evaluated for rates of death, myocardial infarction (MI) and stent thrombosis (ST) at 4 years from their index procedure. The number of evaluable patients was 2157 and 1918 for the 12- and 24-month landmarks, respectively. In both landmark analyses, there was a significantly lower 4-year rate of death/MI in the group that continued DAPT compared to the group that did not (12-month: 10.5% vs. 14.5%, p=0.01; 24-month: 5.7% vs. 8.6%, p=0.02). Beneficial differences in the group that continued on DAPT were preserved after multivariate and propensity adjustment. There were no significant differences in definite stent thrombosis in either landmark analysis. In conclusion, at 12-months and 24-months following DES implantation, continued use of DAPT, was associated with lower 4-year risk of death and myocardial infarction.
Keywords: Coronary disease, Stents, Mortality, Thrombosis
INTRODUCTION
The optimal duration of dual antiplatelet therapy (DAPT) after implantation of drug-eluting stents (DES) is unclear.1–3 Based upon the initial randomized clinical trials of the first-generation DES, the Food and Drug Administration and ACC/AHA Guidelines initially recommended DAPT for 6 months with paclitaxel-eluting (Taxus) stents and 3 months with sirolimus-eluting (Cypher) stents.4–6 Because of studies that suggested that patients who received 1-year of clopidogrel therapy after DES implantation had better survival at 2-years compared to those who received therapy for a shorter duration, current guidelines now recommend at least 1-year of DAPT for patients who receive a DES if they are not at high risk for bleeding many advocated for extending the duration of DAPT.7–12 Subsequently, several reports revealed conflicting information regarding the benefit of DAPT beyond one year after DES implantation.3, 13–15 Other recent data from the PRODIGY and EXCELLENT trials suggest that shorter DAPT duration may be safe in selected patients. 14 Since the duration of DAPT is still largely driven by individual physician and/or patient preference rather than evidence, we sought to determine the effect of varying durations of DAPT in unrestricted clinical practice upon 4-year rates of death, myocardial infarction (MI), and stent thrombosis after drug-eluting stent implantation.
METHODS
The National Heart, Lung and Blood Institute (NHLBI) Dynamic Registry is a multicenter North American registry that has been described in detail previously.16 Each center received institutional review board approval. Five recruitment waves of approximately 2000 patients each have been enrolled and were followed. Only Waves 4 5 are included in these analyses since these were the waves in which DES were available. Patients in these two waves were recruited in 2004 and 2006, respectively.
Patients who underwent successful implantation of at least one DES during their index PCI and who were discharged on clopidogrel and aspirin were considered eligible for analysis (Supplemental Figure 1). Patients who reported DAPT discontinuation and subsequently resumed it at a later time-point in follow-up were excluded. Patients who received a combination of bare-metal stents (BMS) and DES were included in the analysis given that the duration of DAPT would be driven by the placement of the DES.
Data on baseline demographic, clinical, angiographic, and procedural characteristics during the index PCI, as well as the occurrence of death, myocardial infarction, and the need for repeat revascularization were collected. At each follow-up time-point, patients were asked to provide information regarding their medications. If the patients discontinued clopidogrel they were asked the reason. With the use of the Social Security Administration’s Death Master File (www.ntis.gov/products/ssa-dmf.asp), coordinators evaluated the vital status of patients who were lost to follow-up. If patients underwent subsequent repeat revascularization (either PCI or CABG), vessel-specific and lesion-specific data were collected whenever possible.
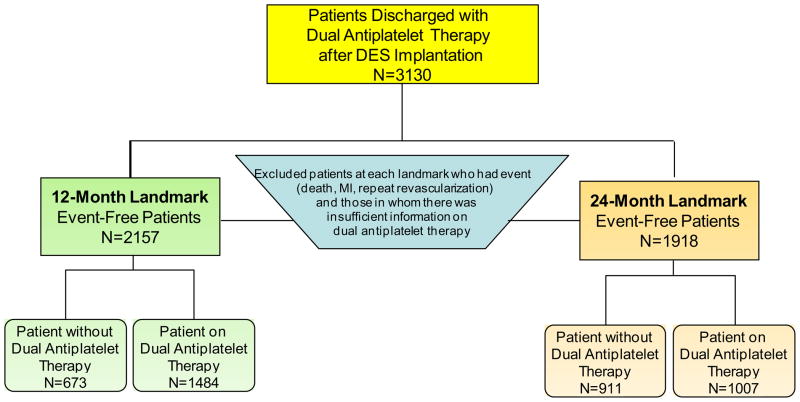
Landmark analysis allows for selecting patients who are “event free” at a specific time point following the index procedure and then following them forward. “Event-free” is defined as absence of death, non-fatal myocardial infarction, or repeat revascularization. We analyzed the data using 12-month and 24-month landmarks (Figure 1) and evaluated outcomes at 4-years from the index procedure stratified by use of DAPT. Individuals in whom there was insufficient information regarding their DAPT at either landmark time point were excluded from the analysis.
Figure 1.
Flow-Diagram of 12-Month and 24-Month Landmark Analyses
The primary endpoints were death and MI and the secondary endpoint was repeat revascularization. 16 At all landmark points, patients were stratified by whether they continued the use of DAPT or not and descriptive statistics were summarized as mean for continuous variables and percentages for categorical variables.
Differences between proportions were assessed by the chi-square test or Fisher’s exact test and continuous variables were compared by Wilcoxon nonparametric tests. Similar methods were used for lesion-level analyses. Unadjusted cumulative event rates for adverse outcomes at three years, for every landmark point, were calculated by the Kaplan–Meier method, plotted and compared using the log rank statistic. Patients who did not experience the outcome of interest were censored at the last known date of contact or at four years if contact extended beyond four years.
The independent associations between DAPT use and 4-year death and death/MI was examined in two ways: (1) Cox proportional hazards methods provided point estimates adjusted for important variables identified by forward stepwise selection (entry P-value criterion of ≤0.15, retain criteria of <0.05). Variables included in the model included demographic ones (age, renal disease, pulmonary disease, history of heart failure, cancer), procedural ones (graft lesions, total occlusions, and calcified lesions), and discharge medications); (2) A propensity score approach was used to balance factors associated with the type of therapy. The estimated propensity score for continuation of DAPT was obtained from the fit of a logistic regression model for which demographic, angiographic, and procedural characteristics as well as discharge medications were considered. The proportionality assumption was assessed and met for all Cox proportional hazards models. Hazard ratios with corresponding 95% confidence intervals are reported. All statistical analyses were performed with SAS, version 9.2, and a two-sided p-value of 0.05 or less was considered for statistical significance.
RESULTS
We identified 3130 patients that received at least one DES and who were discharged on DAPT with aspirin and clopidogrel. For the 1-year landmark analysis (Figure 1), 973 patients were not included in the analysis because they either experienced an adverse event during the first year following their index PCI [death 95, nonfatal MI 120, or repeat revascularization 242] or there was insufficient information regarding DAPT use. As a result, the 12-month landmark group included 2157 patients with 1484 (69%) who were continuing their DAPT use at 1 year and 673 (31%) who were not. For the 24-month landmark analysis (Figure 1), 1212 of the 3130 DES-treated patients discharged with aspirin and clopidogrel were not included in the analysis because they either suffered an event [death 185, nonfatal MI 154, or repeat revascularization 327] during the two years following their index PCI or there was insufficient information regarding DAPT use. Accordingly, the 24-month landmark group included 1918 patients with 1007 (53%) who were continuing their DAPT use at 2 years and 911 (47%) who had discontinued DAPT by 2 years. At the 12 month landmark, the subjects that continued DAPT included 24% of the Wave 4 subjects and 44% of Wave 5 subjects. At the 24 month landmark, the subjects that continued DAPT included 17% of the Wave 4 subjects and 35% of Wave 5 subjects.
Table 1 provides baseline characteristics for each landmark assignment. The mean age of the group that continue DAPT was slightly but significantly lower than the group that did not continue DAPT. For both landmark time points, patients on DAPT had a higher prevalence of diabetes and hyperlipidemia, although these differences were small. For both analyses, patients on DAPT more often had a history of prior PCI.
Table 1.
Baseline Clinical, Demographic, Lesion and Procedural Patient Characteristics at 12-month and 24-month Landmark Timepoints.
| 12-Month Landmark | 24-Month Landmark | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Off DAPT (n=673) | On DAPT (n=1484) | p-value | Off DAPT (n=911) | On DAPT (n=1007) | p-value | |
|
Baseline Clinical and Demographics
| ||||||
| Mean Age (Years) | 65 | 63 | 0.003 | 64 | 63 | 0.02 |
| Women | 36% | 32.1% | 0.03 | 34% | 32% | 0.35 |
| White | 80% | 75% | 0.15 | 81% | 75.1% | 0.005 |
| Diabetes Mellitus | 31% | 34.4% | 0.1 | 30% | 34% | 0.06 |
| Hypertension | 76% | 78.2% | 0.32 | 74% | 78% | 0.05 |
| Hypercholesterolemia | 77% | 79% | 0.36 | 76% | 79% | 0.08 |
| Smoking (Current or Former) | 64% | 65% | 0.74 | 65% | 63% | 0.69 |
| Renal Disease | 9.9% | 7% | 0.04 | 8% | 7% | 0.34 |
| Cerebrovascular Disease | 7% | 7% | 0.68 | 6% | 7% | 0.13 |
| Cancer | 8.2% | 6% | 0.11 | 8% | 6% | 0.03 |
| Peripheral Vascular Disease | 6% | 8.1% | 0.14 | 6% | 8% | 0.14 |
| Prior Myocardial Infarction | 22% | 24% | 0.3 | 22% | 25% | 0.12 |
| Prior Percutaneous Coronary Intervention (PCI) | 25% | 35% | <0.001 | 26% | 35% | <0.001 |
| Mean Left Ventricular Ejection Fraction | 54% | 52.9% | 0.64 | 54% | 54% | 0.5 |
|
| ||||||
|
Lesion Characteristics and Procedural Information
| ||||||
| Procedure Indication | 0.003 | 0.03 | ||||
| Acute Myocardial Infarction | 26% | 28% | 27% | 28% | ||
| Unstable Angina Pectoris | 36% | 32% | 34% | 31% | ||
| Stable Angina Pectoris | 19% | 23% | 20% | 23% | ||
| Other | 19% | 17% | 19% | 18% | ||
| Number of Vessels with >70% Stenosis | 0.008 | 0.005 | ||||
| 1 | 55% | 47% | 54% | 47% | ||
| 2 | 28% | 31% | 29% | 32% | ||
| 3 | 16% | 19% | 16% | 19% | ||
| Circumstances of Procedure | <0.001 | 0.008 | ||||
| Elective | 54% | 60% | 55% | 61% | ||
| Urgent | 37% | 29% | 35% | 28% | ||
| Emergent | 9% | 12% | 11% | 11% | ||
| Glycoprotein IIb/IIIa Inhibitor Use | 36% | 34% | 0.47 | 37% | 35% | 0.48 |
| PCI Information | ||||||
| Mean number of DES stents | 1.5 | 1.6 | 0.01 | 1.5 | 1.6 | 0.02 |
| Patients treated with >1 DES | 35% | 40.8% | 0.01 | 36% | 41.3% | 0.02 |
| Patients treated with BMS and DES | 6% | 5% | 0.14 | 5.5% | 4.5% | 0.22 |
| PCI of Proximal LAD | 16.6% | 15.8% | 0.37 | 16.9% | 15% | 0.22 |
| PCI of Left Main | 1.5% | 3.3% | 0.02 | 2% | 3% | 0.15 |
| PCI of Bifurcation Lesions | 11% | 9% | 0.36 | 10% | 10% | 0.78 |
| Mean Number of Lesions Attempted | 1.3 | 1.4 | 0.26 | 1.3 | 1.4 | 0.35 |
| Mean Reference Vessel Size (mm) | 3 | 3 | 0.73 | 3 | 3 | 0.25 |
| Mean Lesion Length (mm) | 16 | 16 | 0.08 | 16 | 16.5 | 0.06 |
| ACC/AHA Classification | 0.79 | 0.24 | ||||
| Type A | 12% | 11% | 13% | 10.5% | ||
| Type B1 or B2 | 63% | 64% | 64% | 63% | ||
| Type C | 25% | 25.3% | 23% | 26% | ||
| Type of Drug-Eluting Stent | ||||||
| Sirolimus | 58% | 60% | 0.34 | 59% | 60% | 0.45 |
| Paclitaxel | 35% | 35% | 0.93 | 35% | 35% | 0.85 |
|
| ||||||
|
Discharge Information
| ||||||
| Bleeding Prior to Discharge | 5% | 4.2% | 0.76 | 4% | 4% | 0.98 |
| Medications on Discharge | ||||||
| Statin | 85% | 85.9% | 0.46 | 85.8% | 86% | 0.98 |
| Beta-blocker | 81% | 82.3% | 0.57 | 80% | 83% | 0.08 |
| ACE-Inhibitor | 52% | 52.4% | 0.86 | 51% | 52.3% | 0.5 |
| Warfarin | 9% | 5% | <0.001 | 8% | 4% | <0.001 |
Baseline clinical variables were based upon patient self-reports or based upon current pharmacologic therapy
For the group that did not continue DAPT, reasons for cessation of clopidogrel was only available in patients from Wave 5 (Supplemental Table 1a and b). In this wave, discontinuation of clopidogrel was physician-mediated in approximately 90% of patients and due to non-compliance in only about 5% of patients.
With respect to lesion and procedural characteristics (Table 1), at both landmarks only minor differences were noted in procedural indications while there was a greater prevalence of multi-vessel CAD and implantation of more than one DES among those patients who continued on DAPT. There were no significant differences at either landmark time point with respect to use of glycoprotein IIb/IIIa inhibitor use, number of lesions attempted, reference vessel size, lesion length or ACC/AHA lesion type classification. There were no differences with type of DES utilized. Procedural success was high in both groups and rates of peri-procedural bleeding were similar. There were no important differences in medications utilized upon discharge, except that more patients who discontinued DAPT earlier had been discharged on coumadin.
For the entire cohort of 3130 patients who were treated with a DES and discharged on DAPT, the 4-year cumulative rates of death, myocardial infarction, repeat revascularization, and stent thrombosis were 9.1%, 7.5%, 18.8%, and 1.4%, respectively.
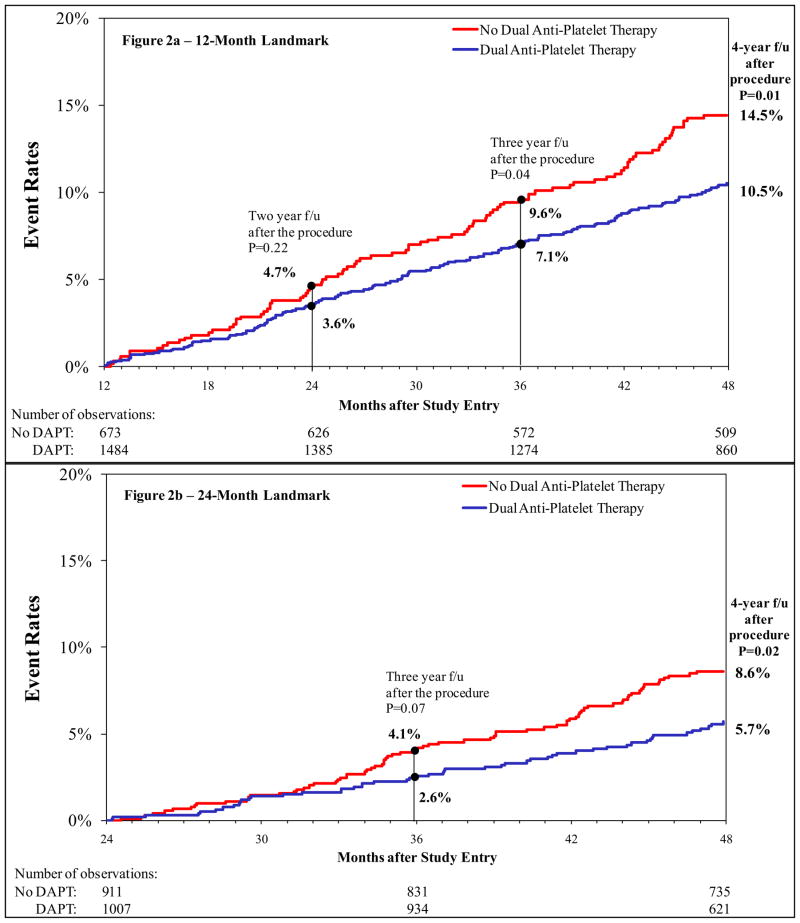
Figure 2a shows the 4-year Kaplan-Meier curves for death and MI for patients in the 12-month landmark analysis. Those who continued on DAPT had a significantly lower risk for 4-year death and MI compared to those who did not (10.5% versus 14.5%, p=0.01). One-year following the landmark, the differences between the 2 groups were small and non-significant; however, the benefit of continuation of DAPT was most appreciated with longer-term follow-up. Overall, for the group that did not continue DAPT, the annual rates of death or myocardial infarction was approximately 4.7%/year while the group that continued on DAPT had an annual event rate of 3.6%/year. The cumulative event rates for the 12-month landmark groups are shown in Table 2a. Risk for death was lower in the group that continued DAPT; however, the rate of repeat revascularization in the 2 years following the landmark was higher in these patients. No significant differences were noted in rates of definite stent thrombosis between the group that continued on DAPT and that did not.
Figure 2.
Kaplan-Meier Curves for Death/Myocardial Infarction for 12-Month (Fig 2a) and 24-Month (Fig 2b) Landmark Time Points
Table 2a.
Cumulative Unadjusted Event Rates for 4-year Follow-up in 12 Month Landmark Group
| Variable | Dual Antiplatelet Therapy at 1 year | ||
|---|---|---|---|
| No (N=911) | Yes (N=1484) | P value | |
| Death | 11.1% | 7.9% | 0.02 |
| Myocardial Infarction | 3.7% | 3.8% | 0.89 |
| Death/Myocardial Infarction | 14.5% | 10.5% | 0.01 |
| Coronary bypass surgery or repeat percutaneous coronary intervention | 6.6% | 11.7% | 0.0002 |
| Stent Thrombosis | 0.2% | 0.8% | 0.11 |
| Coronary artery bypass surgery | 1.6% | 1.9% | 0.65 |
| Percutaneous coronary intervention after discharge | 5.2% | 10.1% | 0.0001 |
Figure 2b shows the 4-year Kaplan-Meier curves for death and MI for the patients in the 24-month landmark analysis. Similar to the observations for the 12-month landmark analysis, patients who continued on DAPT at 24-months experienced less 4-year death and myocardial infarction compared to those who discontinued DAPT by 24-months (5.7% versus 8.6%, p= 0.02). Consistent with the observations in the 12-month landmark analysis, the benefit of DAPT was most appreciable with longer term follow-up. In the 24-month landmark, for the group that did not continue DAPT, the annual rates of death or MI was approximately 4.2%/year while the group continuing DAPT had an annual event rate of 2.8%/year of death and MI. The cumulative event rates for the 24-month landmark groups are shown in Table 2b. Again there was a significantly lower risk for death in the group that continued DAPT (4.1% versus 6.9%, p=0.007). Similar to the 12-month landmark, there were significant differences in rates of repeat revascularization between the 2 groups in the 2 years following this landmark. There were no significant differences in stent thrombosis between the groups.
Table 2b.
Cumulative Unadjusted Event Rates for 4-year Follow-up in 24 Month Landmark Group
| Variable | Dual Antiplatelet Therapy at 2 years | ||
|---|---|---|---|
| No (N=911) | Yes (N=1007) | P value | |
| Death | 6.9% | 4.1% | 0.0065 |
| Myocardial Infarction | 2.0% | 2.0% | 0.94 |
| Death/Myocardial Infarction | 8.6% | 5.7% | 0.02 |
| Coronary bypass surgery or repeat percutaneous coronary intervention | 4.6% | 7.9% | 0.005 |
| Stent Thrombosis | 0.1% | 0.6% | 0.12 |
| Coronary artery bypass surgery | 1.1% | 1.0% | 0.83 |
| Percutaneous coronary intervention after discharge | 3.6% | 7.0% | 0.001 |
As seen in Figure 3, the adjusted Cox 4-year mortality for patients who continued on DAPT at 12-months compared to those who did not was lower (HR 0.72, 95% CI 0.53–0.98, p=0.04) and there was a 28% relative risk reduction for the combined endpoint of death and myocardial infarction (HR 0.72, 95% CI 0.55–0.93, p=0.01). The adjusted risk for 4-year death in the 24-month landmark assignment was also lower (HR 0.60, 95% CI 0.40–0.91, p=0.02) with a 36% reduction in death/MI (HR 0.64, 95% CI 0.45–0.92, p=0.01). We also performed a propensity-adjusted Cox analysis (Supplemental Figure 2), which confirmed the association between continued DAPT and the adjusted relative risk reduction for all-cause mortality (12-month landmark: HR 0.77, 95% CI 0.57–1.04, p=0.09; 24-month landmark: HR 0.57, 95% CI 0.38–0.87, p=0.009) and the combined outcome of death/MI (12-month landmark: HR 0.74, 95% CI 0.57–0.97, p=0.03; 24-month landmark: HR 0.64, 95% CI 0.44–0.91, p=0.01). Overall, continued dual DAPT showed benefit across many subgroups (Supplemental Table 2), and we could not identify any subgroup in which longer DAPT was hazardous.
Figure 3.
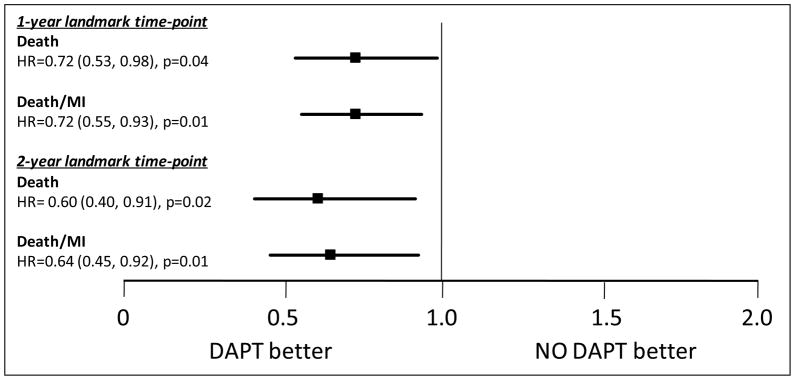
Adjusted relative risk for Death and Death/MI with Dual Anti-platelet Therapy at 12- and 24-Month Landmark Time Points
DISCUSSION
Our study showed that, among patients treated with DES, there was a significant benefit in all-cause mortality and the combined outcome of death and MI associated with continued use of DAPT compared to the discontinuation of DAPT at both the 12-month and the 24-month landmark time points.
Our findings are in contrast to those of Park, Harjai, and Shin showing that DAPT use beyond 12 months following DES implantation was not associated with any reduction in mortality.13, 14, 17 Their findings may have differed from ours for several reasons. First, Park’s study was derived from merged data of two separate trials in which there was an initial assumption of a 50% relative risk reduction in cardiac death and MI and an expected 5% event rate. However, both trials had an exceptionally lower than expected number of events with rates of death of only 0.5% and 1.5% at follow-up periods of 12-months 24-months, respectively. Accordingly, Park’s study may have been underpowered to detect differences between prolonged versus shorter DAPT use. Moreover, only 25% of the patients from this study had 24-month follow-up which may have limited their ability to detect differences in late outcomes. In the analyses by Harjai and Shin, although the rates for death and MI were slightly higher than in Park’s study, these rates were still lower than observed in our report. Our patient sample is derived from unrestricted DES use and may be more reflective of general clinical practice. Also, the number of patients in these 2 studies who received a DES was relatively small with only 1024 patients and 844 patients, respectively.14, 17 We observed mortality rates substantially higher than seen in these studies; the overall 4-year all-cause mortality of 9.1% reported in our study following DES implantation was consistent with other large studies with similar follow-up periods.18–20
Our results extend the findings from several previous reports which showed a benefit with less death and MI associated with treating patients with DAPT beyond 1 year following DES implantation.12, 21 A reduction in death was the primary benefit of extended aspirin and clopidogrel. The Duke Registry also found a modest reduction in nonfatal myocardial infarction consistent with the strong trend in our report. Our results reinforce these prior findings and extend them by demonstrating a significant death/MI benefit of continued DAPT beyond 2 years following DES placement.
One proposed rationale for extended duration of DAPT is to prevent DES thrombosis, which may be associated with both MI and death.10, 22 We observed no significant differences in rates of definite stent thrombosis based on DAPT duration, and this is consistent with other studies.12, 13 However, our study was certainly not designed to be powered for detection of differences in stent thrombosis given its rarity. Our results are in agreement with findings from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial and the Percutaneous Coronary Intervention-Clopidogrel in Unstable Angina to Prevent Recurrent Events (PCI-CURE) trial which showed a benefit to 1-year of clopidogrel therapy following PCI.23, 24
There are some limitations to our analysis. First, our study is an observational registry, and although we adjusted for several variables, it is possible that residual confounding could account for the observed differences between longer duration of DAPT use and shorter durations. For example, we did not have data detailing anemia or bleeding, features known to be associated with higher mortality. Patients with these conditions may be less likely to receive prolonged DAPT.25, 26 Second, we did not have information delineating the exact time and reason for cessation of DAPT in each subject. As a result, we could not link the clinical events with the specific time of cessation of dual antiplatelet therapy. Finally, we only evaluated the first generation of DES given timing of our enrollment. The ongoing Dual Antiplatelet Therapy study will lend further insight into the nature of the benefit of prolonged DAPT use, the impact upon rare events such as stent thrombosis, the differences across the present-day array of DES, and the effect upon bleeding outcomes.27
Supplementary Material
Acknowledgments
SOURCES OF FUNDING:
This study was supported by grants (HL-33292-12 through HL-33292-22) from NHLBI.
Footnotes
DISCLOSURES:
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.King SB, 3rd, Smith SC, Jr, Hirshfeld JW, Jr, Jacobs AK, Morrison DA, Williams DO, Feldman TE, Kern MJ, O’Neill WW, Schaff HV, Whitlow PL, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW 2005 Writing Committee Members. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation. 2008;117:261–295. doi: 10.1161/CIRCULATIONAHA.107.188208. [DOI] [PubMed] [Google Scholar]
- 2.Grines CL, Bonow RO, Casey DE, Jr, Gardner TJ, Lockhart PB, Moliterno DJ, O’Gara P, Whitlow P. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation. 2007;115:813–818. doi: 10.1161/CIRCULATIONAHA.106.180944. [DOI] [PubMed] [Google Scholar]
- 3.Airoldi F, Colombo A, Morici N, Latib A, Cosgrave J, Buellesfeld L, Bonizzoni E, Carlino M, Gerckens U, Godino C, Melzi G, Michev I, Montorfano M, Sangiorgi GM, Qasim A, Chieffo A, Briguori C, Grube E. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation. 2007;116:745–754. doi: 10.1161/CIRCULATIONAHA.106.686048. [DOI] [PubMed] [Google Scholar]
- 4.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, Jacobs AK, Kern MJ, King SB, 3rd, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:156–175. doi: 10.1161/CIRCULATIONAHA.105.170815. [DOI] [PubMed] [Google Scholar]
- 5.<http://www.fda.gov/cdrh/PDF2/P020026.html>.
- 6.<http://www.fda.gov/cdrh/pdf3/p030025.html>.
- 7.Ong AT, Hoye A, Aoki J, van Mieghem CAG, Rodriguez Granillo GA, Sonnenschein K, Regar E. Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J Am Coll Cardiol. 2005;45:947–953. doi: 10.1016/j.jacc.2004.09.079. [DOI] [PubMed] [Google Scholar]
- 8.Ong AT, McFadden EP, Regar E, de Jaegere PP, van Domburg RT, Serruys PW. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol. 2005;45:2088–2092. doi: 10.1016/j.jacc.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 9.Regar E, Lemos PA, Saia F, Degertekin M, Tanabe K, Lee CH, Arampatzis CA, Hoye A, Sianos G, de Feyter P, van der Giessen WJ, Smits PC, van Domburg RT, Serruys PW. Incidence of thrombotic stent occlusion during the first three months after sirolimus-eluting stent implantation in 500 consecutive patients. Am J Cardiol. 2004;93:1271–1275. doi: 10.1016/j.amjcard.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 11.Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 12.Eisenstein EL, Anstrom KJ, Kong DF, Shaw LK, Tuttle RH, Mark DB, Kramer JM, Harrington RA, Matchar DB, Kandzari DE, Peterson ED, Schulman KA, Califf RM. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159–168. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 13.Park SJ, Park DW, Kim YH, Kang SJ, Lee SW, Lee CW, Han KH, Park SW, Yun SC, Lee SG, Rha SW, Seong IW, Jeong MH, Hur SH, Lee NH, Yoon J, Yang JY, Lee BK, Choi YJ, Chung WS, Lim DS, Cheong SS, Kim KS, Chae JK, Nah DY, Jeon DS, Seung KB, Jang JS, Park HS, Lee K. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362:1374–1382. doi: 10.1056/NEJMoa1001266. [DOI] [PubMed] [Google Scholar]
- 14.Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, Castriota F, Colombo F, Tebaldi M, Fucà G, Kubbajeh M, Cangiano E, Minarelli M, Scalone A, Cavazza C, Frangione A, Borghesi M, Marchesini J, Parrinello G, Ferrari R. Short-versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–2026. doi: 10.1161/CIRCULATIONAHA.111.071589. [DOI] [PubMed] [Google Scholar]
- 15.Brar SS, Kim J, Brar SK, Zadegan R, Ree M, Liu ILA, Mansukhani P, Aharonian V, Hyett R, Shen AYJ. Long-term outcomes by clopidogrel duration and stent type in a diabetic population with de novo coronary artery lesions. J Am Coll Cardiol. 2008;51:2220–2227. doi: 10.1016/j.jacc.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 16.Marroquin OC, Selzer F, Mulukutla SR, Williams DO, Vlachos HA, Wilensky RL, Tanguay JF, Holper EM, Abbott JD, Lee JS, Smith C, Anderson WD, Kelsey SF, Kip KE. A comparison of bare-metal and drug-eluting stents for off-label indications. N Engl J Med. 2008;358:342–352. doi: 10.1056/NEJMoa0706258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin DH, Chae IH, Youn TJ, Cho S, Kwon DA, Suh JW, Chang HJ, Cho YS, Chung WY, Choi YJ, Gwon HC, Han KR, Choi DJ. Reasonable duration of Clopidogrel use after drug-eluting stent implantation in Korean patients. Am J Cardiol. 2009;104:1668–1673. doi: 10.1016/j.amjcard.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 18.Mauri L, Silbaugh TS, Wolf RE, Zelevinsky K, Lovett A, Zhou Z, Resnick FS, Normand ST. Long-term clinical outcomes after drug-eluting and bare-metal stenting in Massachusetts. Circulation. 2008;118:1817–1827. doi: 10.1161/CIRCULATIONAHA.108.781377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shishehbor MH, Goel SS, Kapadia SR, Bhatt DL, Kelly P, Raymond RE, Galla JM, Brener SJ. Long-term impact of drug-eluting stents versus bare-metal stents on all-cause mortality. J Am Coll Cardiol. 2008;52:1041–1048. doi: 10.1016/j.jacc.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Morice MC, Serruys PW, Barragan P, Bode C, VanEs GA, Stoll HP, Snead D, Lauri L, Cutlip DE, Sousa E. Long-term clinical outcomes with sirolimus-eluting coronary stents: five-year results of the RAVEL trial. J Am Coll Cardiol. 2007;50:1299–1304. doi: 10.1016/j.jacc.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Petersen JL, Barron JJ, Hammill BG, Cziraky MJ, Anstrom KJ, Wahl PM, Eisenstein EL, Krucoff MW, Califf RM, Schulman KA, Curtis LH. Clopidogrel use and clinical events after drug-eluting stent implantation: findings from the HealthCore Integrated Research Database. Am Heart J. 2010;159:462–470. doi: 10.1016/j.ahj.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Spertus JA, Kettelkamp R, Vance C, Decker C, Jones PG, Rumsfeld JS, Messenger JC, Khanal S, Peterson ED, Bach RG, Krumholz HM, Cohen DJ. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113:2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 23.Steinhubl SR, Berger PB, Mann JT, 3rd, Fry ET, DeLago A, Wilmer C, Topol EJ CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 24.Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA Cure Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 25.Aronow HD, Steinhubl SR, Brennan DM, Berger PB, Topol EJ. Bleeding risk associated with 1 year of dual antiplatelet therapy after percutaneous coronary intervention: Insights from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2009;157:369–374. doi: 10.1016/j.ahj.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Berger PB, Bhatt DL, Fuster V, Steg G, Fox KAA, Shao M, Brennan DM, Hacke W, Montalescot G, Steinhubl SR, Topol EJ. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation. 2010;121:2575–2583. doi: 10.1161/CIRCULATIONAHA.109.895342. [DOI] [PubMed] [Google Scholar]
- 27.The Dual Antiplatelet Therapy study: critical path drives unique collaboration to improve patient safety. Silver Spring, MD: Food & Drug Administration; ( http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/SpotlightonCPIProjects/ucm171900.htm.) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.