Abstract
To elucidate the role of the hippocampus in unaware relational memory, the present study examined the performance of amnesic patients with medial temporal lobe (MTL) lesions on a cued category-exemplar generation task. In contrast to a prior study in which amnesic patients showed impaired performance (Verfaellie et al., Cognitive, Affective, and Behavioral Neuroscience, 2006, 6, 91–101), the current study employed a task that required active processing of the context word at test. In this version of the task, amnesic patients, like control participants, showed enhanced category exemplar priming when the context word associated with the target at study was reinstated at test. The finding of intact implicit memory for novel associations following hippocampal lesions in a task that requires flexible use of retrieval cues is inconsistent with a relational memory view that suggests that the hippocampus is critical for all forms of relational memory, regardless of awareness. Instead, it suggests that unaware memory for within-domain associations does not require MTL mediation.
Keywords: Amnesia, Relational memory, Implicit memory, Associative priming, Medial temporal lobes, Hippocampus
1. Introduction
There is broad agreement that the hippocampus is critical for relational learning mechanisms that allow the rapid encoding of associations between different aspects of an event (Cohen & Eichenbaum, 1993; Eichenbaum, Yonelinas, & Ranganath, 2007; Henke, 2010). Such relational learning affords memories flexibility, in that the distinct elements and their interrelations can be retrieved through a variety of retrieval cues. This flexibility is an essential feature of declarative or explicit memory, and perhaps not surprisingly, much of the research characterizing the role of the hippocampus in relational memory has focused on explicit memory (Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001).
More controversial is the question as to whether the hippocampus also plays a role in the formation of relational representations that do not require conscious access (implicit memory). Several studies have reported impaired implicit memory in amnesia for visual scenes (Ryan, Althoff, Whitlow, & Cohen, 2000; Ryan & Cohen, 2004) or elements in a spatial array (Chun & Phelps, 1999), suggesting that the hippocampus is critical for relational memory even when the products of memory are unavailable to conscious awareness (Henke, 2010; Ryan & Cohen, 2003). However, this conclusion has been challenged on grounds that performance on these tasks may depend on explicit memory (Smith, Hopkins, & Squire, 2006; Smith & Squire, 2008) or is impaired only when lesions extend beyond the hippocampus (Manns & Squire, 2001). From this perspective, it has been argued that the hippocampus does not contribute to unaware (implicit) relational memory.
This debate may be informed by studies of implicit memory for novel verbal associations in amnesia, i.e., the association between two words that do not have a pre-existing link. By the former (relational) view, amnesic patients should show impaired performance on such tasks, on the assumption that these tasks depend on the formation of flexible relational representations. By the latter view, amnesic patients should show normal performance on such tasks, as long as performance in control participants is not “contaminated” by explicit memory. The results of these sorts of studies have been mixed. Amnesic patients have shown intact implicit memory for novel associations on tasks such as perceptual identification (Gabrieli, Keane, Zarella, & Poldrack, 1997), lexical decision (Goshen-Gottstein, Moscovitch, & Melo, 2000), and word reading (Moscovitch, Winocur, & McLachlan, 1986), but impaired performance on a cued word stem completion task (Cermak, Bleich, & Blackford, 1988; Mayes & Gooding, 1989; Schacter & Graf, 1986b; Shimamura & Squire, 1989).
Based on this evidence, we previously postulated that the hippocampus might be critical for implicit memory for novel conceptual, but not perceptual, associations (Verfaellie & Keane, 2001, 2002). Priming for novel conceptual associations requires establishment of a meaningful relationship at study between two words that do not have a pre-existing semantic link (Schacter & Graf, 1986a). If one supposes that implicit memory for novel conceptual (but not perceptual) associations requires the establishment of flexible relational representations, this account may be consistent with the relational view. Because evidence regarding the status of conceptual associative priming came from a single task, however, and because that task does not always yield reliable priming even in control subjects (Cermak et al., 1988), we explored this hypothesis further by examining amnesics’ performance on two other conceptual associative priming tasks (Verfaellie, Martin, Page, Parks, & Keane, 2006). Consistent with our hypothesis, we found impaired associative priming in a cued category-exemplar task in which the reinstatement of contextual information associated with a target at study could enhance the likelihood of target generation at test. Importantly, performance on this task was dissociated from explicit memory in control participants, suggesting that the deficit in amnesia was truly one of unconscious memory. Inconsistent with our hypothesis, however, we found intact associative priming in a relatedness judgment task. These findings suggested that a simple perceptual/conceptual distinction could not account for the pattern of spared and impaired implicit associative memory in amnesia.
However, a relational memory account of our findings may be plausible in the context of closer consideration of the differences between these two conceptual associative tasks. One potentially important difference lies in the nature of the retrieval cues. In the relatedness judgment task, memory is tested using the exact retrieval cues that are present during learning (i.e., the two words are presented together in the study phase as well as in the test phase). In the category exemplar task, by contrast, a novel retrieval probe is presented at test. More specifically, whereas the context word and target word are presented together in the study phase (e.g., mall-rain), only the context word is presented at test together with a not previously seen category probe (e.g., mall-weather phenomena?). Thus, whereas associative priming on the relatedness judgment task can be mediated by unitized representations consisting of a rigid blend of the individual elements – representations that can be formed directly within neocortical regions (Goshen-Gottstein et al., 2000; Moses & Ryan, 2006) –, associative priming in the category exemplar task requires representational flexibility. As such, it can be argued that only performance on the exemplar task reflects true relational learning. Consequently, amnesics’ failure to show associative priming in the category exemplar task might be taken as support for the notion that the hippocampus is critical for relational learning, regardless of whether those memories are accessible to awareness.
Before accepting this interpretation, however, it is important to consider another possible reason for the discrepancy in amnesics’ performance in the two conceptual associative priming tasks. In the relatedness judgment task, processing of the first word is mandatory, whereas in the category exemplar task, processing of the first word of the pair (the context word) is incidental to the task. Amnesics’ impaired implicit associative memory in the latter task might be due to a failure to process the context word spontaneously (Verfaellie et al., 2006).
To provide further evidence regarding the role of the hippocampus in implicit relational memory, the current study examined the performance of patients with MTL amnesia on a cued category-exemplar task in which processing of the context word was mandatory. As outlined above, a critical feature of the task is that the retrieval probes at test are not present during study. This ensures that memory is mediated by the formation of a relational representation in which the individual components are maintained, rather than by a rigid, unitized representation. If amnesic patients show impaired associative priming in this task, it would provide critical evidence that the hippocampus is essential for unaware relational memory. Alternatively, if amnesic patients show intact associative priming in this task, it would suggest that some forms of relational representation can be established without hippocampal mediation.
2. Methods
2.1. Participants
Nine MTL-amnesic patients (4 female) and 18 healthy controls (15 female) participated in this study1. The neuropsychological profiles of all patients indicated severe impairment limited to the domain of memory (see Table 1). The control group was matched to the amnesic group in terms of age (mean = 64 years), education (mean = 15 years), and WAIS-III Verbal IQ (mean = 107.1; all ts < 1).
Table 1.
Amnesic patient demographic and neurological characteristics.
| Patient | Etiology | Age | Edu | WAIS, III | WMS, III | Volume z | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VIQ | GM | VD | AD | WM | Hippoc. | Ant. parahipp. | Post. parahipp. | ||||
| P01 | Encephalitis | 54 | 14 | 92 | 45 | 56 | 55 | 85 | −7.59 | −.377 | −1.74 |
| P02 | Encephalitis | 65 | 12 | 106 | 69 | 68 | 77 | 111 | −6.69 | −5.03 | −2.04 |
| P03 | Anoxia/Ischemia | 59 | 12 | 83 | 52 | 56 | 55 | 91 | N/A | N/A | N/A |
| P04 | Pulmonary arrest | 50 | 14 | 90 | 45 | 53 | 52 | 93 | −7.54 | −.59 | .52 |
| P05 | CO poisoning | 52 | 14 | 111 | 59 | 72 | 52 | 96 | −2.35 | −.54 | 1.46 |
| P06 | Encephalitis | 80 | 18 | 135 | 45 | 53 | 58 | 141 | N/A | N/A | N/A |
| P07 | Cardiac arrest | 56 | 17 | 134 | 70 | 75 | 67 | 126 | N/A | N/A | N/A |
| P08 | Ischemia | 55 | 18 | 119 | 73 | 75 | 55 | 104 | N/A | N/A | N/A |
| P09 | Cardiac arrest | 59 | 16 | 110 | 62 | 68 | 61 | 92 | N/A | N/A | N/A |
Note: Age = Age in years; Edu = Education in years; WAIS, III = Wechsler Adult Intelligence Scale, III; VIQ = Verbal IQ; WMS, III = Wechsler Memory Scale, III; GM = General Memory; VD = Visual Delayed; AD = Auditory Delayed; WM = Working Memory; Hipp. = Hippocampus; Ant. Parahipp. = Anterior Parahippocampus, which includes temporal pole, enthorhinal, and perirhinal cortex; Post. Parahipp. = Posterior Parahippocampus.
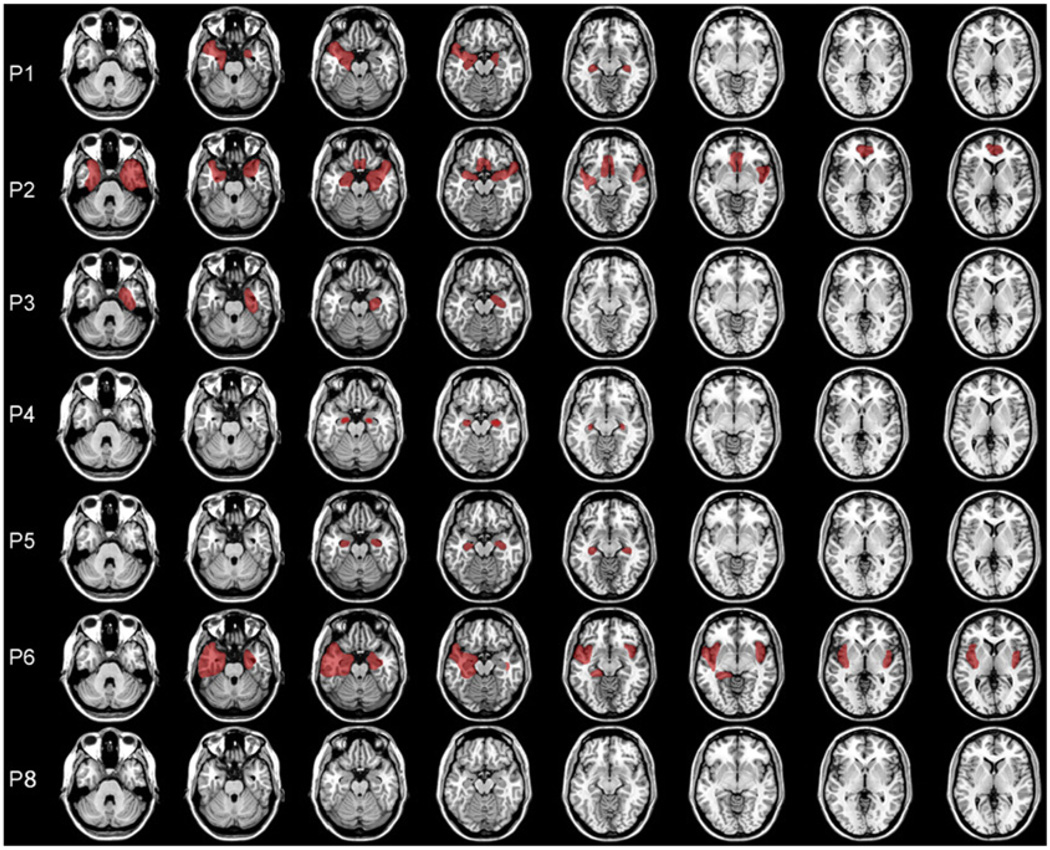
Etiology of amnesia was herpes encephalitis in three patients and ischemic or anoxic event in six patients. MR/CT scans confirmed medial temporal lobe pathology for all but two patients (P07 and P09) who could not be scanned because they had a pacemaker. For these patients, MTL pathology was inferred on the basis of etiology and neuropsychological profile. Of the patients who were scanned, six had visible lesions and one had reduced hippocampal size, but no visible lesion (P08). Lesion reconstruction overlays are presented in Fig. 1. Volumetric data were available for four patients (for details of volumetric analysis, see Kan, Giovanello, Schnyer, Makris, & Verfaellie, 2007). Two of these patients showed volume reductions (> 2 SD below the mean of eight age- and gender-matched controls subjects) limited to the hippocampus (P04 and P05), and two had volume reductions in the hippocampus and surrounding parahippocampal gyrus (see Table 1). The hippocampus was the only structure damaged in all patients and no common volume reductions were found outside the MTL.
Fig. 1.
Reconstruction of lesions based on CT and MRI scans.
2.2. Materials
Stimuli consisted of 2 exemplars from each of 36 categories, which were selected with the constraint that they not be the most often generated exemplar for their category. According to the normative database of Van Overschelde et al. (2004), the proportion of participants who generated the exemplar was .47 averaged across exemplars. Two lists were created, each consisting of one exemplar from each of the 36 categories. These lists formed the targets for the implicit and explicit tasks, and assignment of list to task was counterbalanced across subjects. Each target was paired with an unrelated word that served as its context word. Two master-lists, each consisting of 36 word pairs thus constructed, were matched in terms of word frequency (Francis & Kucera, 1982) of the target word (mean = 52 vs 68), and context word (mean = 40 vs 53).
Each master list was divided into three sets of 12 word pairs, which were rotated through the three experimental conditions (old, recombined, new). These three sets were also matched in terms of word frequency of the target and of the context word. Three distinct study forms were created for each master list. Each study form consisted of two sets of 12 items (old and recombined items) to which a participant was exposed during the study phase. For the set of items constituting the old condition, the pairs were kept intact. For the set of items constituting the recombined condition, the context words were randomly recombined with other target words from that set. Pairs were presented pseudorandomly within a study form, with the proviso that no more than three items from the same set occurred in a row. Two filler pairs were added to the beginning and end of the study list. There was one test form to which all participants were exposed in the test phase. Each test form consisted of 36 trials; on each trial a context word was presented with the category label corresponding to the target word with which the context word was paired in the master list. In this manner, in the old condition, context words were presented at test with the category probe corresponding to the target word with which the context word was paired at study. In the recombined condition, context words were presented at test with a category probe corresponding to an exemplar paired with a different context word at study. In the new condition, neither the context word at test nor any exemplar of the category probe had been seen in the study phase.
2.3. Procedure
During the study phase of both the implicit and explicit task, 24 word pairs were presented one at the time on the screen, with the context word presented above the target word (e.g., mall-rain). Participants were asked to read the pair aloud, and then heard a sentence that related both words (e.g., “Most people go to a mall when there is rain”). They were asked to indicate how believable the statement was on a 3-point scale. Participants responded verbally, and 1500 msec after their response, the next trial was initiated.
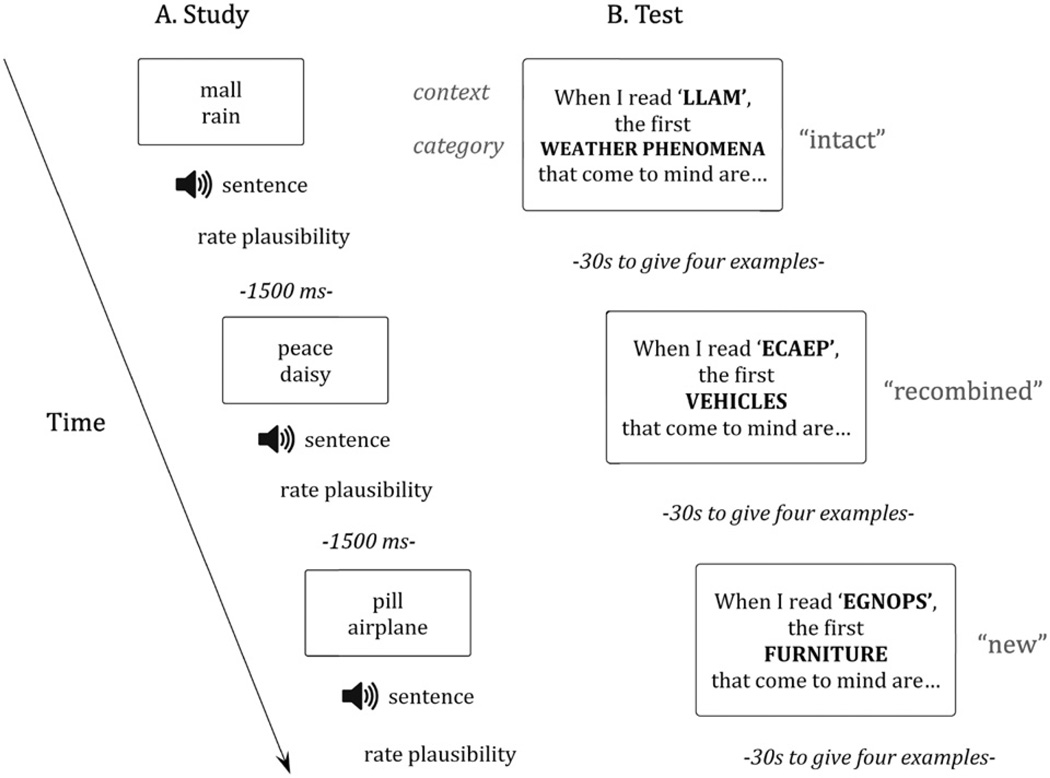
During the implicit test phase, on each trial the participant was asked to generate 4 exemplars of a category in response to a context word paired with the name of a category. To ensure active processing of the context word, that word was presented backwards (e.g., llam), and the participant had to decipher it before the category probe was presented on the screen. On each trial, participants read aloud a visually-presented sentence that took the following form: “when I read [context word spelled backwards], the first [category name] that come to mind are …” (see Fig. 2). The sentence was presented in two parts to ensure that the participant had deciphered the context word before the category probe was presented. For example, a participant might see and read “when I hear the word ‘LLAM’, the first //WEATHER PHENOMENA that come to mind are …”. After the participant had read the first segment (and deciphered the context word), the second segment was added.
Fig. 2.
Experimental Design. (A) Participant read word pair; sentence was then read aloud and the participant rated its plausibility. (B) The participant saw on the screen, “When I read [context word backwards] the first [category name] that come to mind are …”. The second half of the sentence was presented after the participant correctly identified the context word. The participant was then given 30 s to come up with four examples from the given category.
During the explicit test phase, on each trial a context word was again presented backwards for the participant to read aloud, followed by a category. The participant was asked to remember if an item from that particular category was on the list of words they had seen earlier, and if so, to provide that exemplar. This was done in the context of a visually presented sentence that took the following form: “when I read LLAM, I remember the following //WEATHER PHENOMENA from the list …#x0201D;. As in the implicit test, the sentence was presented in two parts, with the second segment added after the context word was deciphered. Participants were told that not all categories had been on the study list and to refrain from answering if they could not remember having seen an exemplar from a particular category.
Control participants completed the implicit and explicit tasks at least 6 weeks apart; amnesic participants completed the two tasks at least 2 weeks apart. The implicit task always preceded the explicit task.
3. Results
3.1. Implicit memory
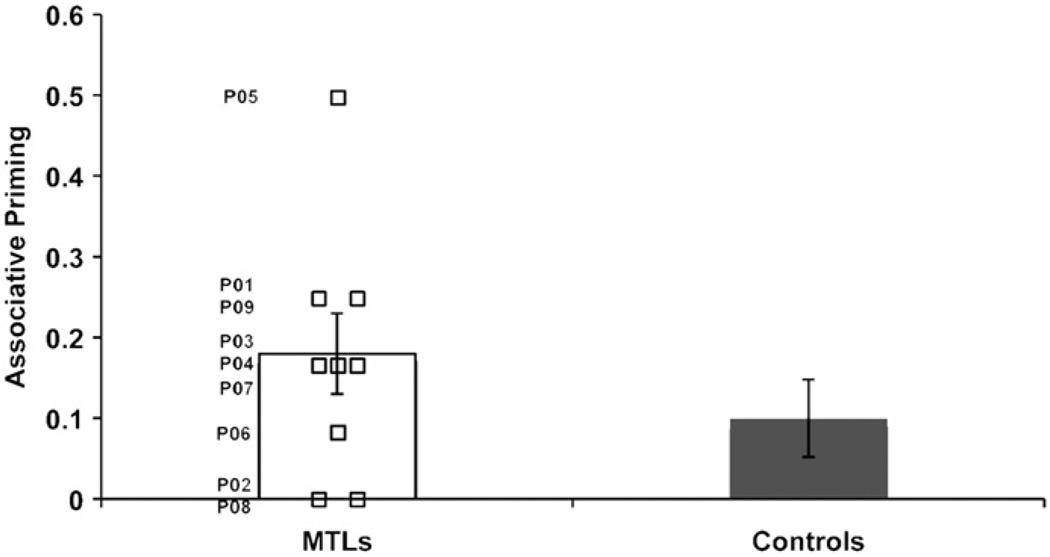
Generation of the target word among the four exemplars was considered a correct response. Table 2 presents response accuracy for the amnesic and control group in the three test conditions. Associative priming was measured as the difference in performance between the old and recombined conditions. Item priming was measured as the difference in performance between the recombined and new conditions. As can be seen, both groups showed associative priming of comparable magnitude, and neither group showed item priming2. Because the focus of this paper is on associative priming, only analyses pertaining to this effect are presented in what follows. Associative priming was evaluated in a mixed factorial ANOVA with group as the between-subjects factor and condition (old, recombined) as the within-subjects factor. This analysis revealed a main effect of condition (F(1,25) = 12.58, p < .01), but no effect of group or group x condition interaction (both Fs < 1). Individual scores for the amnesic patients are plotted in Fig. 3. As can be seen, one patient (P05) was an outlier in terms of magnitude of priming. Even when this patient’s data were excluded, associative priming in the amnesic group (mean = .14) was still equivalent to that in controls (mean = .10).
Table 2.
Mean proportion (and standard error) of target words generated (implicit memory) and recalled (explicit memory) by amnesic patients and controls as a function of condition.
| Group | Condition | Implicit | Explicit |
|---|---|---|---|
| Amnesic | Old | 0.61 (.08) | 0.20 (.04) |
| Recombined | 0.44 (.06) | 0.22 (.05) | |
| New | 0.48 (.05) | 0.06 (.02) | |
| Control | Old | 0.61 (.03) | 0.55 (.07) |
| Recombined | 0.51 (.03) | 0.37 (.06) | |
| New | 0.50 (.04) | 0.00 (.00) |
Fig. 3.
Associative priming in individual amnesic patients and in controls. Associative priming reflects the difference in the proportion of target words generated in the old compared to the recombined condition.
In a secondary analysis, we analyzed the output position of the target when it was generated as one of the responses, on the assumption that reinstatement of the context word may lead to preferential sampling of the target item over other activated items (Kinoshita, 1999). This analysis revealed a marginal effect of condition (F(2,50) = 2.41, p = .10), with targets in the old condition output at an earlier position (mean = 2.04) than those in the recombined condition (mean = 2.21), which in turn were output at an earlier position than those in the new condition (mean = 2.36). There was no effect of group or group x condition interaction (both Fs < 1), suggesting that output order effects were similar in the two groups.
Because it has recently been suggested that the perirhinal cortex plays a critical role in conceptual priming (Wang, Lazzara, Ranganath, Knight, & Yonelinas, 2010), we separately examined associative priming in the 4 patients with documented damage of the parahippocampal gyrus including perirhinal cortex (P01, P02, P03, and P06). Associative priming in this subgroup (mean =.11) was similar in magnitude to that observed in the control group (mean =.09), suggesting that associative conceptual priming does not depend on the integrity of perirhinal cortex.
3.2. Explicit memory
Table 2 also presents the proportion of targets recalled by amnesic and control participants in the three test conditions. Target responses in the new condition, representing baseline guessing, were minimal in both groups. As expected, cued recall was lower in the amnesic than in the control group, and only the control group showed better recall when the cue word had previously been seen together with the to-be-remembered target (old condition) than when it had been studied with another target (recombined condition). A mixed factorial ANOVA with group as the between-subjects variable and condition (old, recombined) as the within-subjects variable revealed a main effect of group (F(1,24) = 7.09, p < .05) and a group x condition interaction (F(1,24) = 5.51, p <.05). Control subjects showed an associative cueing effect (old > recombined; t (17) = 3.55, p < .01) but amnesic patients did not (t < 1).
4. Discussion
The finding that amnesic patients with MTL damage show intact implicit memory in a cued category-exemplar generation task provides evidence that not all forms of unaware relational memory depend on the integrity of the hippocampus. Performance on the current task required the establishment of novel verbal associations that could be expressed flexibly, by way of retrieval cues at test that differed from those presented at study, yet patients showed a benefit associated with the reinstatement of context information that was equivalent to that seen in nonamnesic control participants. These findings suggest that the amnesic impairment in implicit memory for novel verbal associations in our earlier study (Verfaellie et al., 2006) was not due to the relational demands of the memory task, but rather, to the fact that patients did not actively focus on processing the context word. In a similar vein, Kinoshita (1999) demonstrated in normal individuals that dividing attention at test – a manipulation that would serve to reduce processing of the context word – eliminated the effect of context reinstatement in a word stem completion task. Additionally, presentation of the context word in mirror reversed fashion may have enhanced reliance on conceptual processing (Graf & Levy, 1984; Tardif & Craik, 1989), thereby facilitating reinstatement of the conceptual link that was formed between the context word and target.
The implications of our findings for memory theory critically depend on the notion that performance in the cued category-exemplar generation task reflects relational memory. Could it be argued that intact implicit memory in the amnesic group did not depend on the formation of a relational representation, but rather, was supported by the creation of a unitized representation? Two factors argue against this alternative view: First, the formation of unitized representations requires that the components are encoded directly, without a mediator (Mayes, Montaldi, & Migo, 2007). In our task, unrelated words were linked through a mediator, by virtue of being meaningfully related in a sentence. Second, even if a unitized representation could be formed (e.g., mall-rain), this would not be sufficient to support performance on the category exemplar task: implicit memory depended on flexible access to the newly formed representation, because only one of the elements of the studied pair (the context word; mall) was presented at test and the other element was replaced by its category label (weather phenomenon). For exemplar generation to be influenced by the context word, maintenance of a flexible representation of the relation between the two items of the studied pair was necessary.
The finding that amnesic patients show intact implicit memory on a relational memory task is inconsistent with the relational memory view, which posits that all forms of relational memory, regardless of awareness, depend on the hippocampus (Henke, 2010; Ryan & Cohen, 2003). Instead, the results point to the need for a more nuanced view that can accommodate the fact that patients with hippocampal lesions show impaired implicit memory for scenes (Ryan et al., 2000; Ryan & Cohen, 2004) and spatial contextual information (Chun & Phelps, 1999), yet show intact implicit memory for verbal associations (the present study).
A possible way to reconcile these findings may be with reference to the distinction between within-domain and cross-domain associations (for a similar distinction in the domain of explicit memory, see Mayes et al., 2007). In this context, the term “domain” refers to specific processing modules, such as visual form, spatial location, lexical information, and semantics. The tasks that have yielded impaired associative priming in amnesia require the establishment of perceptual associations between domains, such as information about visual form and spatial location. Because these different types of information are processed in distant neocortical regions, the formation of cross-domain associations may depend on informational convergence in the hippocampus. By contrast, within-domain perceptual associations (e.g., between two visual word forms) can be formed within closely adjacent and interacting neurons within a single neocortical region, and thus may not require hippocampal mediation. Analogously, conceptual associations may be formed within closely adjacent neurons in lateral temporal regions involved in the processing of semantic information. Because of this spatial proximity, hippocampal mediation likewise may not be necessary.
The hypothesis that unaware memory for cross-domain associations, but not within-domain associations, is hippocampally dependent rests on the assumption that performance on cross-domain associative priming tasks indeed reflects unaware memory. As noted earlier, however, this is a matter of controversy. With regard to studies of scene memory, Squire and colleagues (Smith et al., 2006; Smith & Squire, 2008) have argued that the eye-movement measures that index relational memory depend on awareness of the relational manipulation. As such, findings of impaired performance in patients with hippocampal lesions (Ryan et al., 2000; Ryan & Cohen, 2004) do not threaten the view that only aware memories depend on the hippocampus (Clark & Squire, 1998; Zola & Squire, 2000). From this perspective, the finding in the present study of intact implicit verbal relational memory in patients with hippocampal lesions could be seen as further support for the notion that unaware memory is not hippocampally dependent, even when such memory is relational in nature.
Manns and Squire (2001) have further suggested that the parahippocampal gyrus, rather than the hippocampus proper, may contribute to some forms of unaware relational memory, such as that evident in the contextual cueing task (Chun, 2000). Our finding that implicit memory for verbal associations was intact not only in patients with hippocampal lesions, but also in patients with extensive MTL lesions encompassing the parahippocampal gyrus, suggests that extra-hippocampal MTL regions do not contribute to all forms of implicit relational memory. It reinforces the point made earlier that the neural basis of unaware relational memory may critically depend on the informational content of the to-be-formed associations.
The fact that patients with extensive MTL lesions (including perirhinal cortex) show normal performance on a conceptual associative priming task is inconsistent with a recent proposal that conceptual priming critically depends on the integrity of perirhinal cortex (Wang et al., 2010). Examining conceptual priming for single items (rather than associations), Wang and colleagues found that perirhinal activation during encoding of words was related to behavioral priming in a category exemplar task, and the same region implicated in the fMRI study showed maximal overlap in patients who had impaired conceptual priming. It should be noted, however, that patients in that study either had undergone temporal lobectomy or had suffered infarcts of the posterior cerebral artery, and thus, also had varying degrees of temporal neocortical damage. While the inconsistency between the current findings and those of Wang et al. (2010) will require further study, the possibility cannot be ruled out that temporal neocortical damage was responsible for patients’ impairment in conceptual priming in Wang et al.’s study.
Our proposal that within-domain associative priming is mediated by the neocortical regions responsible for the processing of such information leads to the suggestion that conceptual associative priming may reflect plasticity in neocortical regions involved in semantic processing. Evidence relevant to this notion comes from a recent fMRI study that compared encoding of word–word and picture–picture pairs and examined the relationship between encoding and subsequent associative recognition (Park & Rugg, 2011). That study found both material-independent subsequent associative memory effects (in the MTL) and material-specific subsequent associative memory effects (in neocortical regions). Specifically, subsequent memory for word–word pairs was associated with enhanced encoding-related activity in a region of the middle temporal gyrus previously implicated in retrieval of semantic information. Thus, encoding of item–item associations was facilitated by enhanced processing in the very regions that support processing of individual items (Summerfield et al., 2006). While Park and Rugg (2011) focused on encoding of novel associations in the context of explicit memory, it stands to reason that implicit memory for novel associations could be similarly mediated.
While the present study speaks to the neural basis of implicit memory for novel conceptual associations, it leaves open questions regarding the functional basis of such priming. We consider two possible ways in which reinstatement of a context word may facilitate performance in the cued category-exemplar task. To consider these possibilities, it is helpful to take as point of departure single item priming. Priming for single items reflects the greater ease of processing or availability of an item that may result from the temporary enhancement of the activation level of its corresponding representation (Dorfman, 1994; Reder, Park, & Kieffaber, 2009). In a standard category-exemplar generation task, for instance, semantic activation from the category label (e.g., weather phenomena) is thought to spread to many exemplars; semantic activation of the target (e.g., rain) is enhanced by its recent encounter in the study list, hence increasing the likelihood that it will be produced as the response. Building on this notion, in a cued category-exemplar priming task, one might postulate that activation spreads from all aspects of the probe, including the context word (e.g., mall). Because an episodic link was established between the context word and the target at study, activation could spread from the context word to the target, thus enhancing its activation and potentially boosting it over that of competitors not associated with the context word (for a similar view, see Reder et al., 2009).
Alternatively, it is possible that the context reinstatement influences priming not by virtue of enhancing the activation level of the target, but rather, by biasing selection among activated candidates towards the target. Kinoshita (1999) offered an interpretation of this sort for context effects in word stem completion. By this view, it is not that the item with the highest activation level is ultimately produced, but rather, that reinstatement of the context word directs attention towards the target with which it was paired at study, in preference over other activated words. Whether an activation or selection mechanism better accounts for the findings in the cued category-exemplar generation task remains to be determined by future studies.
Acknowledgements
This research was supported by NIMH grant 57681 and by the Medical Research Service of the Department of Veterans Affairs. The authors thank Amanda Utevsky and Matt Cruz for research assistance.
Footnotes
One patient was available for the implicit task, but not for the explicit task.
The absence of item priming in this task may be due to the fact that reading the context word backwards served as a divided attention task. Several studies have shown that item priming in a category generation task is reduced (Gabrieli et al., 1999; Mulligan, 1997) or eliminated (Mulligan, 1997; Mulligan & Hartman, 1996) under conditions of divided attention. Alternatively, as suggested by a reviewer, the failure to observe item priming may reflect the relatively high baseline level of performance in the new condition.
References
- Cermak LS, Bleich RP, Blackford SP. Deficits in the implicit retention of new associations by alcoholic Korsakoff patients. Brain and Cognition. 1988;7:312–323. doi: 10.1016/0278-2626(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Chun MM. Contextual cueing of visual attention. Trends in Cognitive Sciences. 2000;4:170–177. doi: 10.1016/s1364-6613(00)01476-5. [DOI] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nature Neuroscience. 1999;2:844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Dorfman J. Sublexical components in implicit memory for novel words. Journal of Experimental Psychology: Learning, Memory and Cognition. 1994;20:1108–1125. doi: 10.1037//0278-7393.20.5.1108. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection. New York: Oxford University Press; 2001. [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency Analysis of English Usage: Lexicon and Grammar. Boston: Houghton Mifflin Co.; 1982. [Google Scholar]
- Gabrieli JDE, Keane MM, Zarella M, Poldrack RA. Preservation of implicit memory for new associations in global amnesia. Psychological Science. 1997;8:326–329. [Google Scholar]
- Gabrieli JDE, Vaidya CJ, Stone MV, Francis WS, Thompson-Shill SL, Fleischman DA, et al. Convergent behavioral and neuropsychological evidence for a distinction between identification and production forms of repetition priming. Journal of Experimental Psychology: General. 1999;128:479–498. doi: 10.1037//0096-3445.128.4.479. [DOI] [PubMed] [Google Scholar]
- Goshen-Gottstein Y, Moscovitch M, Melo B. Intact implicit memory for newly formed verbal associations in amnesic patients following single study trials. Neuropsychology. 2000;14:570–578. doi: 10.1037//0894-4105.14.4.570. [DOI] [PubMed] [Google Scholar]
- Graf P, Levy BA. Remembering: conceptual and perceptual processing involved in reading rotated passages. Journal of Verbal Learning and Verbal Behavior. 1984;23:405–424. [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nature Reviews Neuroscience. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Kan IP, Giovanello KS, Schnyer DM, Makris N, Verfaellie M. Role of the medial temporal lobes in relational memory: neuropsychological evidence from a cued recognition paradigm. Neuropsychologia. 2007;45:2589–2597. doi: 10.1016/j.neuropsychologia.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S. Priming for novel associations: evidence for an attentional component. Memory. 1999;7:385–404. [Google Scholar]
- Manns JR, Squire LR. Perceptual learning, awareness, and the hippocampus. Hippocampus. 2001;11:776–782. doi: 10.1002/hipo.1093. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Gooding P. Enhancement of word completion priming in amnesics by cueing with previously novel associates. Neuropsychologia. 1989;27:1057–1072. doi: 10.1016/0028-3932(89)90185-1. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G, McLachlan D. Memory as assessed by recognition and reading time in normal and memory-impaired people with Alzheimer’s disease and other neurological disorders. Journal of Experimental Psychology: Learning, Memory and Cognition. 1986;6:1068–1074. doi: 10.1037//0096-3445.115.4.331. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ryan JD. A comparison and evaluation of the predictions of relational and conjunctive accounts of hippocampal function. Hippocampus. 2006;16:43–65. doi: 10.1002/hipo.20131. [DOI] [PubMed] [Google Scholar]
- Mulligan NW. Attention and implicit memory tests: the effects of varying attentional load on conceptual priming. Memory and Cognition. 1997;25:11–17. doi: 10.3758/bf03197281. [DOI] [PubMed] [Google Scholar]
- Mulligan NW, Hartman M. Divided attention and indirect memory tests. Memory and Cognition. 1996;24:453–465. doi: 10.3758/bf03200934. [DOI] [PubMed] [Google Scholar]
- Park H, Rugg MD. Neural correlates of encoding within- and across-domain inter-item associations. Journal of Cognitive Neuroscience. 2011;23:2534–2543. doi: 10.1162/jocn.2011.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reder LM, Park H, Kieffaber PD. Memory systems do not divide on consciousness: reinterpreting memory in terms of activation and binding. Psychological Bulletin. 2009;135:23–49. doi: 10.1037/a0013974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychological Science. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Cohen NJ. Evaluating the neuropsychological dissociation evidence for multiple memory systems. Cognitive, Affective and Behavioral Neuroscience. 2003;3:168–185. doi: 10.3758/cabn.3.3.168. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Cohen NJ. Processing and short-term retention of relational information in amnesia. Neuropsychologia. 2004;42:497–511. doi: 10.1016/j.neuropsychologia.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Graf P. Effects of elaborative processing on implicit and explicit memory for new associations. Journal of Experimental Psychology: Learning, Memory and Cognition. 1986a;12:432–444. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Graf P. Preserved learning in amnesic patients: perspectives from research on direct priming. Journal of Clinical and Experimental Neuropsychology. 1986b;8:727–743. doi: 10.1080/01688638608405192. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. Impaired priming of new associations in amnesia. Journal of Experimental Psychology: Learning, Memory and Cognition. 1989;15:721–728. doi: 10.1037//0278-7393.15.4.721. [DOI] [PubMed] [Google Scholar]
- Smith CN, Hopkins RO, Squire LR. Experience-dependent eye movements, awareness, and hippocampus-dependent memory. Journal of Neuroscience. 2006;26:11304–11312. doi: 10.1523/JNEUROSCI.3071-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CN, Squire LR. Experience-dependent eye movements reflect hippocampus-dependent (aware) memory. Journal of Neuroscience. 2008;28:12825–12833. doi: 10.1523/JNEUROSCI.4542-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Greene M, Wager T, Egner T, Hirsch J, Mangels JA. Neocortical connectivity during episodic memory formation. PLoS Biology. 2006;4:855–886. doi: 10.1371/journal.pbio.0040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif T, Craik FIM. Reading a week later: perceptual and conceptual factors. Journal of Memory and Language. 1989;28:107–125. [Google Scholar]
- Van Overschelde JP, Rawson KA, Dunlosky J. Category norms: an updated and expanded version of the Battig and Montague (1969) norms. Journal of Memory and Language. 2004;50:289–335. [Google Scholar]
- Verfaellie M, Keane MM. Scope and limits of implicit memory in amnesia. In: De Gelder B, De Haan E, Heywood C, editors. Unconscious Minds. Oxford: Oxford University Press; 2001. pp. 151–162. [Google Scholar]
- Verfaellie M, Keane MM. Impaired and preserved memory processes in amnesia. In: Squire LR, Schacter DL, editors. Neuropsychology of Memory. Third ed. New York: Guilford Press; 2002. [Google Scholar]
- Verfaellie M, Martin E, Page K, Parks E, Keane MM. Implicit memory for novel conceptual associations. Cognitive, Affective and Behavioral Neuroscience. 2006;6:91–101. doi: 10.3758/cabn.6.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Lazzara MM, Ranganath C, Knight RG, Yonelinas AP. The medial temporal lobe supports conceptual implicit memory. Neuron. 2010;68:835–842. doi: 10.1016/j.neuron.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, Squire LR. The medial temporal lobe and the hippocampus. In: Tulving E, Craik FIM, editors. The Oxford Handbook of Memory. New York: Oxford University Press; 2000. [Google Scholar]