Figure 1.
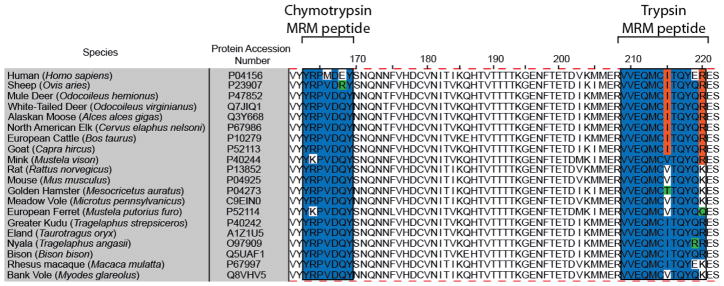
Amino acid alignment of PrP residues 161 to 222 (human PrP numbering) from 20 mammalian species either susceptible to natural prion infection or used as laboratory models. Note that the amino acid sequence for the chymotrypsin MRM peptide is more highly conserved than that for the trypsin-derived MRM peptide. The alignment was performed using the ClustalW algorithm within Jalview software.
