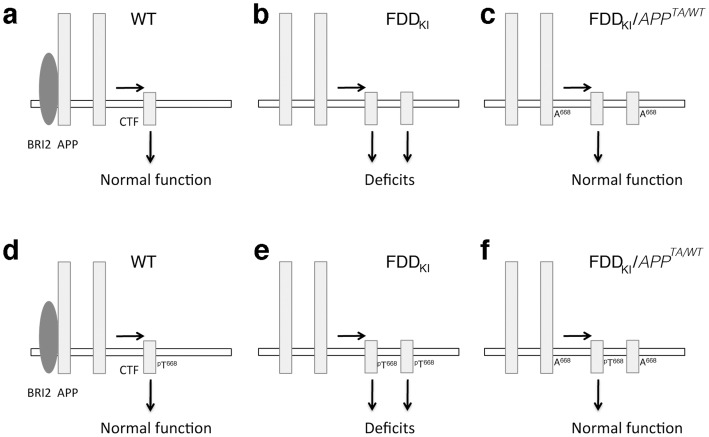
Figure 4. Model depicting the mechanisms by which Thr668 may lead to memory and synaptic plasticity deficits.
(a and b), Due to loss of BRI2 protein, APP processing is increased during synaptic transmission and memory acquisition in FDD leading to increased production of ß-CTF. This event compromises synaptic plasticity and memory acquisition leading to memory deficits. (c), Thr668 is essential for the pathogenic role of ß-CTF, as shown by the evidence that mutating this residue into an Ala prevents development of memory/synaptic deficits. (d–f), Phosphorylation of Thr668 may be required or facilitate the synaptic-toxic role of ß-CTF, since the Thr668Ala mutation prevents phosphorylation.