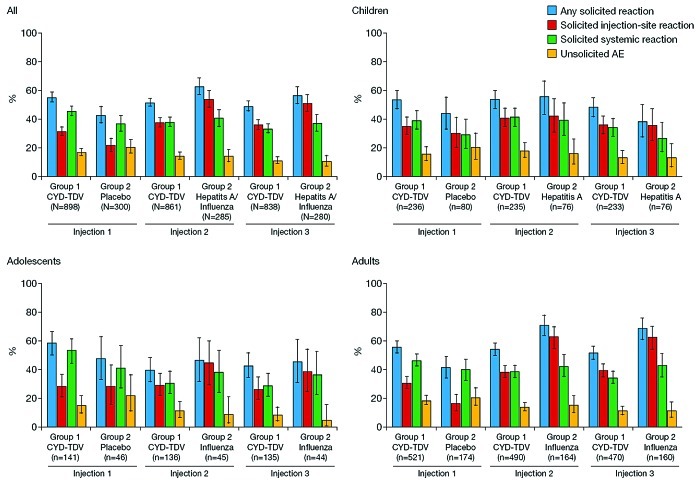
Figure 2. Age-specific reactogenicity of CYD-TDV: proportion of participants by age and vaccine group with different categories of adverse events after each vaccination. Control group participants aged < 12 y at inclusion received intramuscular doses of hepatitis A vaccine and those aged ≥ 12 y received subcutaneous inactivated influenza vaccine.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.