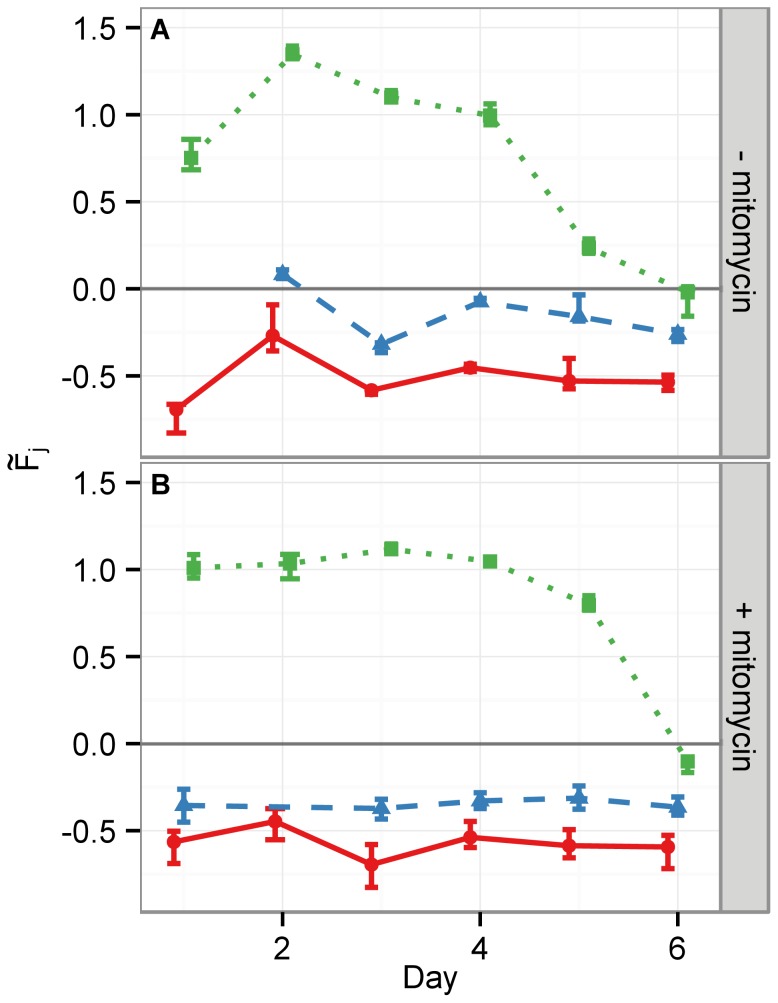
Figure 3. Median responses of the five independent FDA classifiers using the hold-out data set.
The error bars depict the 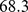 % c.i. Panels (A) and (B) show the FDA classifier response (
% c.i. Panels (A) and (B) show the FDA classifier response (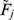 ) to cells without or with mitomycin treatment, respectively. The lines are shown to guide the eye and depict the measurement medians for the different treatment conditions. CTL: red circles and solid lines, NGF: green squares and dotted lines, EGF: blue triangles and dashed lines. The horizontal line through the origin
) to cells without or with mitomycin treatment, respectively. The lines are shown to guide the eye and depict the measurement medians for the different treatment conditions. CTL: red circles and solid lines, NGF: green squares and dotted lines, EGF: blue triangles and dashed lines. The horizontal line through the origin  marks the decision threshold. FDA scores larger than
marks the decision threshold. FDA scores larger than  correspond to the differentiated cell status while scores below correspond to undifferentiated cells. Only on day
correspond to the differentiated cell status while scores below correspond to undifferentiated cells. Only on day  EGF treated without mitomycin in panel (A) are slightly above
EGF treated without mitomycin in panel (A) are slightly above  , such that approximately
, such that approximately 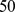 % of the cells under this condition are falsely classified as differentiated. However, NGF treated cells are always far away from the decision threshold
% of the cells under this condition are falsely classified as differentiated. However, NGF treated cells are always far away from the decision threshold  within the first four days. Hence, a more conservative threshold would remedy the false decision for EGF on day
within the first four days. Hence, a more conservative threshold would remedy the false decision for EGF on day  while still correctly classifying NGF treated cells.
while still correctly classifying NGF treated cells.