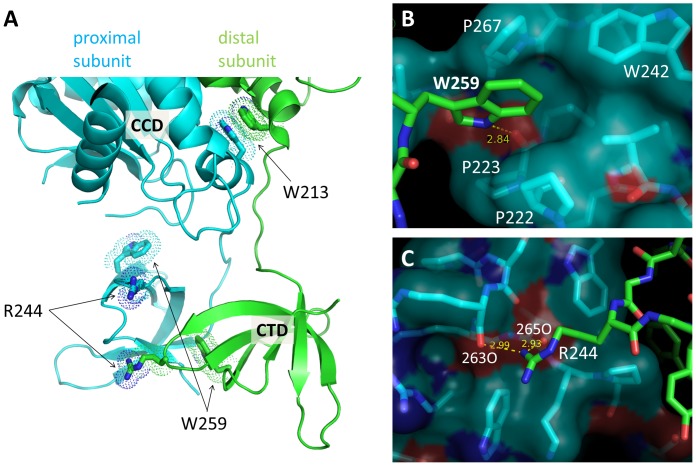
Figure 5. Positions of the amino acid residues around the RSV IN dimer interface mutated in this study.
A) The CCD-CTD dimer of RSV IN, with W213, R244, and W259 side chains from both subunits shown in sticks. B) A close-up view of W259 and the surrounding residues P222, P223, W242, and P267 at the CTD-CTD interface. W259 is inserted into a hydrophobic pocket where it also forms a hydrogen-bond with a backbone carbonyl group of P223. C) A close-up view of the salt bridges formed by R244 from the green subunit in (A) at the CTD-CTD interface.