Table 1.
Substrate scope.
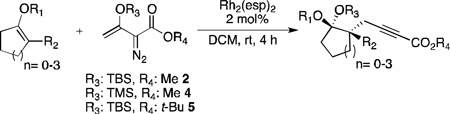 | ||||
|---|---|---|---|---|
| Entry | Substrate | Diazo | Product | Yield[a] [%] |
| 1 | 4 | 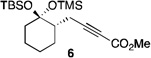 |
49[b] | |
| 2 | 2 |  |
79[b] | |
| 3 | 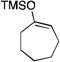 |
2 | 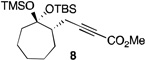 |
61[b] |
| 4 | 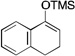 |
2 | 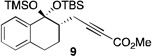 |
69[b] |
| 5 | 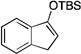 |
2 | 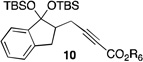 |
33[c] |
| 6 | 5 | 84[d] | ||
| 7 | 2 | 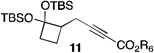 |
51[c,e] | |
| 8 | 5 | 98[d] | ||
| 9[f] | 2 | 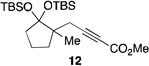 |
56 | |
| 10[f] | 2 | 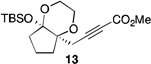 |
53[b] | |
Isolated yield.
d.r. > 20:1.
R4=Me.
R4=t-Bu.
Yield refers to the isolated alkynoate product which was separated from the cyclopropanated byproduct by column chromatography.
Reaction was run at reflux.
