Figure 1. Cross-linking of extracellular matrix proteins by tissue transglutaminase-regulated transamidation.
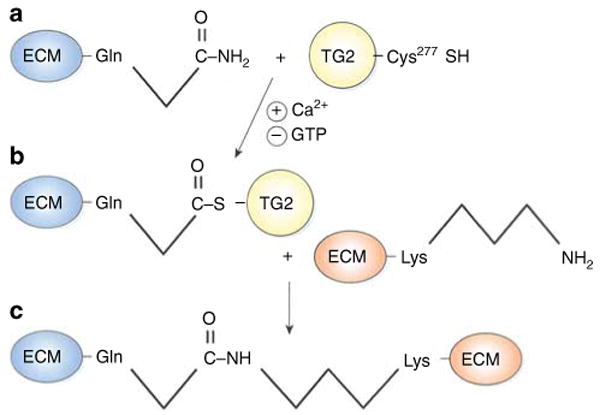
(a) Secreted tissue transglutaminase (TG2), which is stabilized by extracellular calcium, forms a thioester bond between a cysteine (Cys277) within its active site and an extracellular matrix (ECM) glutamine (Gln) residue on its substrate <2>. Both substrate–TG2 binding and TG2 activity are enhanced by calcium and inhibited by guanosine triphosphate (GTP <3>). (b) The TG2–substrate intermediate then interacts with another ECM molecule, and TG2 catalyzes an acyl transfer reaction to form an isopeptide linkage between Gln and lysine (Lys) residues on adjacent ECM proteins (c). The net effect of enhanced TG2 activity is that multiple ECM proteins become glued together to form sclerotic scars <4> <5>.
