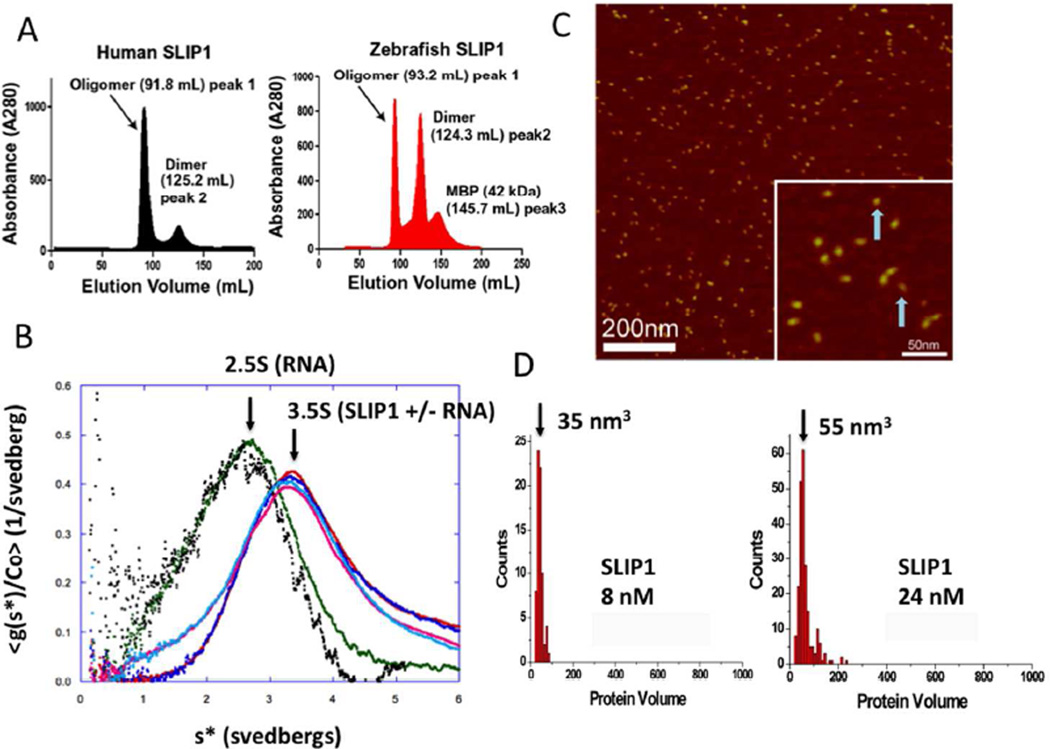
Figure 1. Human SLIP1 is a novel homodimer that does not bind RNA.
In (A) the gel filtration profiles for hSLIP1 and the zebrafish orthologue called MIF4Gdb on an S200 column (GE) is shown. hSLIP1 and MIF4Gdb migrate as higher order oligomers as well as at the apparent molecular weight of the dimer (Mr ~52 kDa). (B) Sedimentation velocity profiles of hSLIP1 in the absence and presence of RNA. The samples were dialyzed against 10 mM potassium phosphate buffer, pH 7.4, 140 mM NaCl, 2.7 mM KCl. For each sample, two different concentrations are shown (0.5 mg/mL and 0.17 mg/mL). Sedimentation velocity profiles for hSLIP1 samples in the presence of a two fold molar excess of histone mRNA stem-loop are shown in cyan and pink. In (C), AFM images of hSLIP1 at 24 nM concentration are shown. Blue arrows point to the two monomers. The hSLIP1 dimers are elongated in shape. Histograms summarizing the volume measurements for the unbound hSLIP1 protein at 8 nM and 24 nM are shown in (D). For the 8 nM sample, N=80, bin size=10. A conversion coefficient of 1.3 was used for globular proteins, which gave the monomer molecular weight of 27kD, corresponding to the SLIP1 monomer. For the 24 nM sample, N=224, bin size=10.