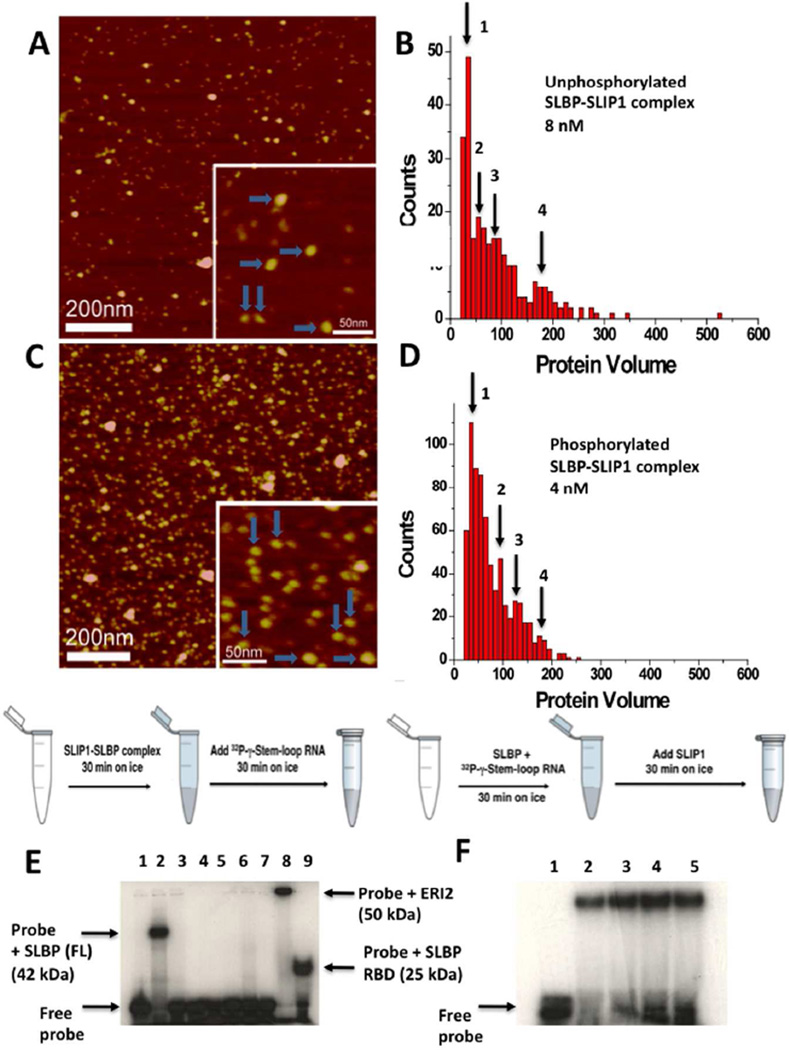
Figure 6. Analysis of the phosphorylated and unphosphorylated hSLBP-hSLIP1 binary and hSLBP-hSLIP1-histone mRNA ternary complexes by AFM and EMSA.
(A) AFM image of the bacterially expressed unphosphorylated hSLBP-hSLIP1 complex at 8 nM. The horizontal blue arrows point to heterotetramers with protein volumes of around 180–200 nm3. The vertical arrows point to heterodimers with a protein volume of around 90 nm3. A histogram summarizing the volume measurements for the complex in (A) is shown in (B). For the 8 nM sample, N=272, bin size=10. (C) AFM image of the baculovirus expressed phosphorylated hSLBP-hSLIP1 complex at 4 nM. The horizontal blue arrows point to heterotetramers with protein volumes of around 170 nm3. The vertical arrows point to heterodimers with a protein volume of around 90 nm3. The image shows an increase in the population of heterodimers relative to heterotetramers in the sample. In (D), a histogram summarizing the volume measurements for the complex in (C) is shown. The concentration of the protein was 4 nM, N=700, and bin size = 10. The four maxima observed in (B) and (D) are summarized in Table 2. (E) EMSA of baculovirus expressed hSLBP and hSLIP1 is shown. The schematic of the reaction was performed is shown above the gel. In all reactions, the histone mRNA probe was added last after the proteins were incubated on ice for 30 min. The free histone mRNA stem-loop probe (final concentration of 0.1 nM) that is labeled at the 5’ end with 32P-γ-ATP is shown in lane 1, the interaction of full length hSLBP (final concentration is 2 mM) with the histone mRNA probe is shown in lane 2. No interaction of hSLIP1 (final monomer concentration is 6 mM) with histone mRNA occurs either in the absence (lane 3) or presence (lane 4) of 5 mM crosslinking agent (DSP). In lanes 5, 6, and 7, hSLBP (2 µM) was incubated with increasing concentrations of hSLIP1 (12 µM, 24 µM, and 36 µM) respectively, and the reactions kept on ice for 30 min. This was followed by addition of 32P-γ-ATP histone stem-loop for another 30 min. No interaction of either SLBP or the complex is observed. Lane 5 shows the interaction of 2 µM ERI2 exonuclease with histone stem-loop RNA (complex Mr ~50 kDa) and lane 6 shows the interaction of hSLBP RBD with histone stem-loop RNA (complex Mr 25 kDa). (F) EMSA showing the interaction of hSLBP with the histone mRNA stem-loop followed by addition of increasing concentrations of hSLIP1. Lane 1 shows the free probe (0.1 nM), lane 2 shows the probe complexed to hSLBP (2 µM). Lanes 3, 4, and 5 show the interaction of the hSLBP-RNA complex with 12 µM, 24 µM, and 36 µM hSLIP1. The hSLIP1 protein was added to a pre-formed SLBP-RNA complex as depicted in the schematic above the gel.