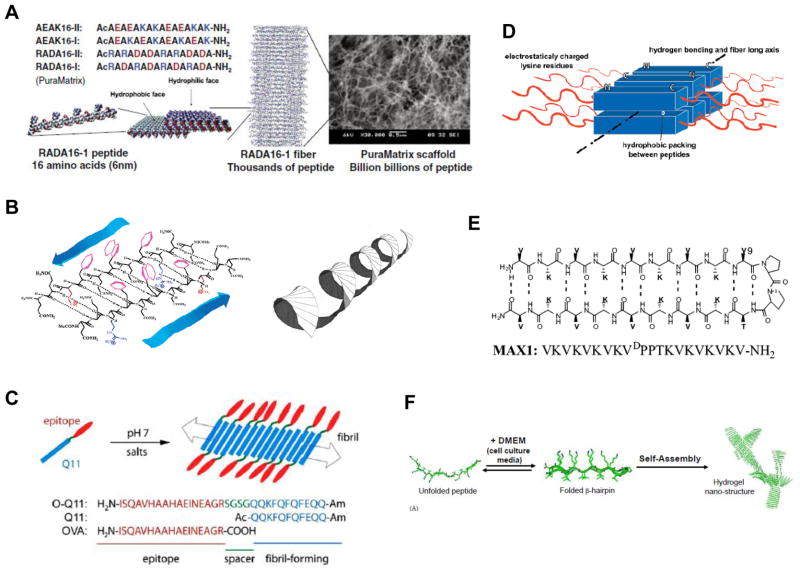
Figure 1. Self-assembling peptides based on the β-sheet.
(A) Schematic of the ionic self-complementary peptides developed by Zhang and coworkers. The peptides pack into sheets and fibers based on hydrophobic interactions on one face of the molecule, and complementary ionic interaction on the other. These fibers then form a highly porous hydrogel. (B) Peptides with alternating hydrophilic and hydrophobic residues assemble into β-sheet structures (left) that form twisted ribbons (right) and bundle into larger fibers. (C) The Q11 peptide (blue) developed by the Collier lab assembles into nanofibers due to β-sheet formation. Appending a peptide epitope (red) results in a high density of the signal on the fibril surface. (D) Amphiphilic tri-block peptides developed by the Hartgerink group. The central hydrophobic block forces self-assembly via hydrophobic between molecules and hydrogen bonding along the fiber axis. Charged lysine residues provide solubility, and balance the hydrophobic forces. (E) The β-hairpin molecule MAX1, which can fold under appropriate conditions and form intramolecular hydrogen bonds. (F) Upon charge screening in cell culture media, the β-hairpin molecule can fold and then subsequently assemble into long nanofibers due to intermolecular interactions. [(A): ref. 7; (B): ref. 21; (C): ref. 28; (D): ref. 29; (E): ref. 32; (F): ref. 38]