Figure 5. Self-assembling short aromatic peptides.
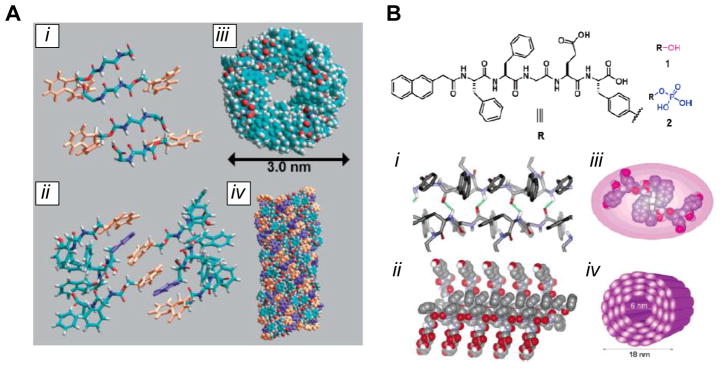
(A) Self-assembly of the Fmoc-FF peptide, as determined by computational modeling. The peptide forms antiparallel β-sheet hydrogen bonds (i); different β-sheets are brought together by π-π stacking between Fmoc groups (orange), with interdigitated phenyl side groups (purple) from the phenylalanine residues (ii); the twist of the β-sheets results in a cylindrical arrangement, shown in top (iii) and side (iv) views. (B) Structure of the Nap-FFGEY peptide, showing the site of phosphorylation that drives the structural transition. The peptide forms a β-sheet structure (i, ii), resulting in further association into nanotube-like structures (iii, iv). [(A): ref. 4; (B): ref. 87]
