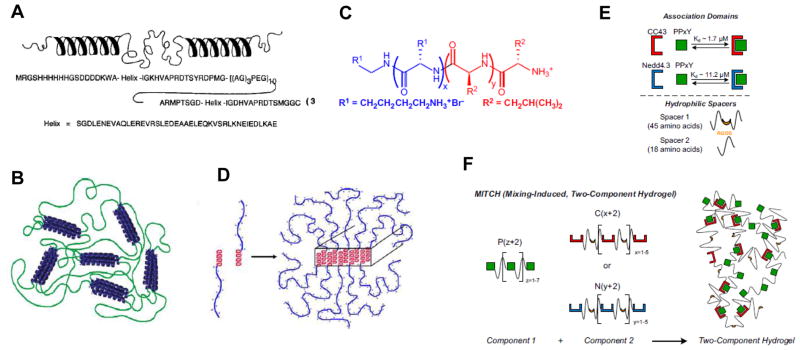
Figure 6. Protein-based self-assembled materials.
(A) Structure of the telechelic, coiled-coil based polypeptide designed by the Tirrell group. (B) Association of the end helices into coiled-coils results in hydrogel formation. (C) Chemical structure of the amphiphilic KxLy block co-polypeptide designed by the Deming laboratory. (D) The leucine α-helices self-assemble due to the hydrophobic effect, forming extended tapes and fibers. (E) Naturally-derived WW domains form a noncovalent complex with PPxY domains. The strength of the interaction between the two can be tuned by changing the specific motif; in this example, the Nedd4.3 variant of the WW domain has a weaker interaction with PPxY than the CC43 variant. (F) Incorporating repeating WW and PPxY domains into separate protein polymers allows for the construction of a hydrogel through protein association interactions. [(A): ref. 92; (B): ref. 97; (C, D): ref. 106; (E,F): ref. 107]