Abstract
Background
Although many time-series studies of ozone and mortality have identified positive associations, others have yielded null or inconclusive results, making the results of these studies difficult to interpret.
Methods
We performed a meta-analysis of 144 effect estimates from 39 time-series studies, and estimated pooled effects by lags, age groups, cause-specific mortality, and concentration metrics. We compared results with pooled estimates from the National Morbidity, Mortality, and Air Pollution Study (NMMAPS), a time-series study of 95 large U.S. urban centers from 1987 to 2000.
Results
Both meta-analysis and NMMAPS results provided strong evidence of a short-term association between ozone and mortality, with larger effects for cardiovascular and respiratory mortality, the elderly, and current-day ozone exposure. In both analyses, results were insensitive to adjustment for particulate matter and model specifications. In the meta-analysis, a 10-ppb increase in daily ozone at single-day or 2-day average of lags 0, 1, or 2 days was associated with an 0.87% increase in total mortality (95% posterior interval = 0.55% to 1.18%), whereas the lag 0 NMMAPS estimate is 0.25% (0.12% to 0.39%). Several findings indicate possible publication bias: meta-analysis results were consistently larger than those from NMMAPS; meta-analysis pooled estimates at lags 0 or 1 were larger when only a single lag was reported than when estimates for multiple lags were reported; and heterogeneity of city-specific estimates in the meta-analysis were larger than with NMMAPS.
Conclusions
This study provides evidence of short-term associations between ozone and mortality as well as evidence of publication bias.
Ozone is a common urban air pollutant with well-documented adverse health effects ranging from respiratory symptoms to increased risk for hospital admissions. The U.S. Environmental Protection Agency (U.S. EPA) establishes primary National Ambient Air Quality Standards (NAAQS) for ozone and other criteria pollutants at a level intended to protect human health with an adequate margin of safety. In 1997, the U.S. EPA proposed adding an ozone standard of 80 ppb based on the daily 8-hour maximum concentration.1 The existing daily 1-hour maximum standard of 120 ppb remains in effect for areas in violation. The changes in regulations were in response to demonstrated effects at concentrations below the existing standard, and evidence that the 8-hour averaging time better represented the time course of the short-term effects of ozone exposure on the respiratory system. The U.S. EPA is required by the Clean Air Act to review the NAAQS at least every 5 years and to revise the standards if needed. At the time of this writing, the EPA has just initiated the development of a new criteria document for ozone. Presently, over 100 million people in the United States live in areas that exceed the 8-hour NAAQS.2
Although many studies have demonstrated the damaging health effects of ozone, studies on mortality have been less consistent. Several time-series studies identified a positive association of ozone concentration with daily mortality counts,3–13 whereas others produced inconclusive evidence, including a negative association, no association, or a positive association that was not statistically significant.14–19 The seemingly conflicting findings of these studies could result from many factors, including chance, variation across the populations, differing analytic methods, and issues related to data quality and measurement error.
Combining information across single-city results is a reasonable approach for estimating an overall effect and for exploring sources of heterogeneity. There are 2 main approaches for combining information. The first is a quantitative meta-analysis of published results. The second is a multicity study in which a uniform analytical framework is applied to time-series data for single cities, and then the city-specific estimates are pooled to generate an overall estimate. These 2 approaches can help resolve controversies from seemingly divergent individual study estimates, increase statistical power, and improve the generalizability of results.
The relationship between ozone and mortality has been examined in several previous meta-analyses, each finding a statistically significant relationship. Recently reported meta-analyses of ozone and mortality include a study by Thurston and Ito,20 which combined results of 16 studies and explored differences in approaches to the modeling of weather; the analysis of Levy et al,21 which used 4 U.S. studies based in Cook County, Illinois, and Philadelphia; the work of Stieb et al,22,23 who extracted results from 109 single- and multicity studies for random effects pooling; and a World Health Organization report that investigated ozone and mortality in Europe.24 In previously conducted multicity time-series studies of ozone and mortality, some researchers found a statistically significant association: studies of 15 European cities,25 23 European cities,26 6 French cities,27 and 80 U.S. cities in one of the National Morbidity, Mortality, and Air Pollution Study (NMMAPS) analyses.28 A positive, but not statistically significant, relationship was identified by Saez et al29 for 7 Spanish cities, and analysis of data from 7 major cities of Korea found a negative, nonstatistically significant association.30 Zmirou et al31 identified a relationship between ozone and cardiovascular and respiratory mortality for 4 cities in western Europe.
NMMAPS initially used mortality data for 90 large U.S. urban communities from 1987 to 1994.28,32–37 Our recent analysis of the extended and updated NMMAPS database for the period 1987 to 2000 included 95 urban centers in the United States and used a uniform statistical framework within each city to estimate a national-average association between short-term changes in ozone and mortality.38 This work investigated multiple model structures, a variety of lag times (including a week-long distributed lag and various single-day lags), several concentration metrics, and potential confounding by particulate matter (PM) and weather such as temperature and dew point temperature. We considered total, cardiovascular, and respiratory mortality and several age categories. City-specific estimates were combined using a Bayesian hierarchical approach to calculate the overall effect of ozone on mortality.
The advantages of either of these approaches over a single-city estimate are the gains in statistical power, the estimation of an overall effect, and the exploration of heterogeneity. However, in the meta-analytic approach, the independently conducted single-city studies generally differ in their statistical models, approaches to addressing confounding by weather and long-term trends, and adjustment for additional pollutants. Meta-analyses are also subject to publication bias; papers reporting a positive association may be more likely to be submitted or accepted for publication. Thus, results of meta-analyses may be biased toward an overestimation of the true effect, although the degree of publication bias is difficult to quantify.
Comparison of results from the meta-analysis and multisite studies provides the opportunity to identify a lower and upper bound for the pooled effect, quantify publication bias, and explore sources of heterogeneity of effects across studies. In this article, we conduct a meta-analysis of 144 estimates from 39 time-series studies of ozone and mortality, published from 1990 to June 2004. By combining information across the time-series studies, we estimate pooled effects by several lags, age groups, cause-specific mortality, location, and concentration metrics. To assess publication bias, we compare the pooled estimates from the meta-analysis with results from NMMAPS.
METHODS
Selection of Studies and Estimates for Meta-Analysis
The time-series studies included in the meta-analysis were systematically selected based on the following criteria:
Studies provided numerical estimates of the relationship between short-term changes in ozone and mortality, as well as an indication of the uncertainty of the central estimate (eg, 95% confidence interval, or t-value).
Studies were peer-reviewed and published in English.
Studies were not based on NMMAPS (to compare meta-analysis results with NMMAPS results).
Studies were published and indexed from 1990 to 21 June 2004.
Estimates were provided for total, cardiovascular, or respiratory mortality.
Studies were not excluded on the basis of other criteria such as adjustment by copollutants; these factors were explored in later analysis. Studies that met our criteria were identified using Pubmed (www.pubmed.com), a service of the National Library of Medicine that includes over 14 million citations. Searches in Pubmed included the words “mortality” or “time-series” in the title or abstract and “ozone” or “O3.” Additional potential references were provided by the U.S. EPA Office of Air Quality Planning and Standards and the Health Effects Institute report of reanalysis of PM and time-series studies.28 If a study was updated such as through newly available statistical techniques or an updated dataset, the most recent results were chosen. For instance, if time-series studies were reanalyzed in response to concerns about the default implementation of generalized additive models (GAM) in S-Plus software,39 we used the reanalysis results. All selected studies addressed potential confounding by temperature.
The authors, as well as other faculty, postdoctoral researchers, and doctoral candidates at the Johns Hopkins Bloomberg School of Public Health, coded the characteristics and results of each selected time-series study. Coding for each time-series study was doublechecked. Investigators of the original studies were contacted to clarify any relevant issues (eg, to ascertain what lag was used).
Only one estimate from each study was included in each meta-analysis result, except when a single study provided results from multiple cities, in which case each city-specific result could be included. Meta-analysis results were not generated if an insufficient number of single estimates (fewer than 4) were available within a particular stratum of results. Estimates of short-term lags were classified as single-day lags of 0 (same day), 1, or 2 days or a 2-day average of lags 0 and 1 or lags 1 and 2. When estimates for multiple lags were provided for a single study, the estimate for lag 0 was used, because this lag was most commonly given. This approach minimizes the bias of choosing the lag with the largest effect, although some studies only presented results for a single lag. If estimates were given for lags 1 and 2 but not for lag 0, the estimate for lag 1 was included. Only estimates based on the whole year’s data were used, except in analyses specifically investigating the warmer time periods. Results are for all ages and without PM adjustment unless otherwise specified.
The selected time-series studies presented results in several forms such as a log-relative rate, the percent increase in mortality, or the regression coefficient— each corresponding to a specified increase in ozone concentrations. The uncertainty of the central estimate was provided as a 95% posterior interval (the Bayesian formulation of the 95% confidence interval), standard error, t-statistic, or ratio of some measure of the central estimate to the standard error. We converted these data to the corresponding log-relative rate (β̂s) with its standard error (), so that multiple studies could be combined in the meta-analysis.
Studies provided results for several concentration metrics. The uncertainty of the central estimate was provided as a 95% interval, standard error, t-statistic, or ratio of some measures of the central estimate to the standard error. We included daily 1-hour and 8-hour maximums calculated for specified time periods that encompassed the daytime but not the whole 24-hour period (eg, 1-hour maximum from 10 am to 8 pm) because the peak ozone levels do not occur at night. Concentration metrics for a specific time period of the day (eg, noon to 8 pm) were not considered because these can differ from the peak average on that day. Results from studies using the 1-hour and 8-hour maximum values were converted to the daily average, except in analyses that specifically addressed comparison across concentration metrics. If information to construct a conversion ratio was provided by the study, this ratio was used. Otherwise, we used a relationship of 20:15:8 for the 1-hour maximum:8-hour maximum:daily average.20 These relationships have been used elsewhere.20 Furthermore, we assumed that 1.96 µg/m3 equals 1 ppb to convert studies into the same metric.
Statistical Methods for Meta-Analysis
We combined information across locations and estimated the pooled effect using a 2-stage Bayesian hierarchical model.40–43 At the first stage, we assumed that the estimated effect β̂s is normally distributed with mean equal to the true effect βs and variance equal to the statistical variance of β̂s, here denoted by vs. At the second stage, we assumed that the true βs is normally distributed with mean µ and between-study variance τ2. The goal of our Bayesian meta-analysis was to estimate the marginal posterior distribution of the pooled effect µ by taking into account the within-city variance (vs), which measures the statistical uncertainty in the estimation of βs and the between-study variance (τ2), which measures the heterogeneity across cities of the true βs. In summary, our model specification can be described as:
| (1) |
We fit model (1) by use of Monte Carlo Markov chain methods44 implemented by the software Winbugs.45 A priori, we assume that µ has a normal distribution with zero mean and very large variance (uninformative or flat prior) and that 1/τ2 has a gamma distribution with shape and scale parameters equal to 0.001 and 0.001. The use of an “uninformative prior” indicates that all possible values of µ are considered approximately equally likely a priori. The goal of a Bayesian analysis is to evaluate the marginal posterior distributions of µ and τ2, where a posterior distribution is defined as the product of the likelihood function times the prior distribution up to a normalizing constant.46
We investigate the sensitivity of the posterior mean of µ to the specification of the prior distribution for the heterogeneity variance τ2. In addition, to investigate the sensitivity of the posterior mean of µ to outliers in the individual reported time-series study estimates, at the second stage, we also assume that the true βs is distributed as a mixture of 2 normal distributions βs | µ, τ2 ~ (1 − p)N(λ1, τ2) + pN(λ2, τ2) where µ = (1 − p)λ1 + pλ2, and that the true βs is distributed as a student-t distribution with 3 degrees of freedom βs | µ, τ2 ~ t3 (µ,τ2).
Multicity Study
In recent NMMAPS analyses,38 we estimated the national-average short-term effect of ozone on mortality by combining information across 95 large U.S. urban communities from 1987 to 2000. The study explored multiple lag structures and model specifications. A generalized linear model with natural cubic splines was used with adjustment for time-varying confounders (weather, seasonality, and long-term trends). A Bayesian hierarchical model was used to combine the city-specific estimates into an overall effect, as shown in equation 1. The statistical models used have been made available at: http://www.ihapss.jhsph.edu/software/NMMAPS/NMMAPS.htm. Full details are reported elsewhere.38
RESULTS
A total of 144 estimates from 39 studies were included in the meta-analysis.3–5,7,9,11–15,18,19,27,29,47–71 We considered the following:
Mortality outcome (total, cardiovascular, or respiratory);
Location (United States or elsewhere);
Potential confounding by PM (no adjustment for PM or adjustment by either PM10 or PM2.5; PM with an aerodynamic diameter no more than 10 or 2.5 µm, respectively);
Cycle of analysis (yearly data or warm periods, eg, summer);
Lag (0, 1, or 2 days; average of days 0 and 1; or average of days 1 and 2);
Age (all ages, or the elderly— either 64+ or 65+); and
Concentration metric (daily average, daily 1-hour maximum, or daily 8-hour maximum).
These same issues were also considered in pooled estimates for NMMAPS.
We performed a chi-squared test for heterogeneity on several subsets of studies, including the United States for total mortality and both the United States and non-United States combined for total mortality. When we rejected the hypothesis of homogeneity, we fitted the 2-stage Bayesian hierarchical model in equation 1 and evaluated the posterior distribution of the pooled effect µ. Table 1 shows posterior means and 95% posterior intervals (the Bayesian formulation of the 95% confidence interval) of µ under alternative distributional assumptions for the second stage and under alternative prior specifications. Note that a single study can contribute multiple estimates if it includes data from more than one city.
TABLE 1.
Sensitivity Analysis Results of the Pooled Log-Relative Rates With Respect to the Specification of the Bayesian Hierarchical Model for Pooling*
| U.S. Only† | U.S. and Non-U.S.‡ | |
|---|---|---|
| II Stage: βs| µ, τ2 ~ N(µ, τ2) | ||
| 1/τ2 ~ Gamma (0.01,0.01) | 0.84 (0.47 to 1.21) | 0.87 (0.55 to 1.19) |
| 1/τ2 ~ Gamma (0.001,0.001) | 0.84 (0.48 to 1.20) | 0.87 (0.55 to 1.18) |
| 1/τ2 ~ Gamma (0.0001,0.0001) | 0.84 (0.49 to 1.19) | 0.87 (0.55 to 1.19) |
| II Stage: βs| µ, τ2 ~ (1 − p)N(λ1, τ2) + pN(λ2, τ2) | ||
| 1/τ2 ~ Gamma (0.001,0.001) | 0.83 (0.42 to 1.24) | 0.96 (0.60 to 1.33) |
| II Stage: βs| µ, τ2 ~ t3 (µ, τ2) | ||
| 1/τ2 ~ Gamma (0.001,0.001) | 0.84 (0.48 to 1.20) | 0.74 (0.47 to 1.00) |
Posterior means and 95% posterior interval (PI), percent increase in mortality per 10-ppb increase in ozone at short-term lags (single-day lags of 0, 1, or 2 days or a 2-day average of lags 0 and 1 or lags 1 and 2.
Eleven city-specific estimates from 9 studies.
Forty-one city-specific estimates from 32 studies.
The pooled estimates are robust to all of these model specifications. Therefore, as a baseline model, we assume at the second stage that βs | µ, τ2 ~ N(µ, τ2) with 1/τ2 ~ Gamma (0.001,0.001). We also explored the findings with respect to a problem with the default implementation of generalized additive models (GAM) in the commonly used statistical software package, S-Plus.39,72 The pooled estimate was larger for studies without GAM problems such as those that used other modeling techniques or used GAM exact.39
Table 2 shows the posterior means and 95% posterior regions of the pooled effects for total, cardiovascular, and respiratory causes separately for U.S. cities only and non-U.S. cities, and for all locations. These pooled effects included time-series studies for short-term lags (defined as lags of 0, 1, or 2 days; or average of either days 0 and 1 or days 1 and 2).
TABLE 2.
Posterior Means and 95% Posterior Intervals of the Pooled Log-Relative Rates for Cause-Specific Mortality*
| U.S. Only |
Non-U.S. Only |
U.S. and Non-U.S. |
||||
|---|---|---|---|---|---|---|
| Posterior Mean (95% PI) |
No. of Estimates, No. of Studies |
Posterior Mean (95% PI) |
No. of Estimates, No. of Studies |
Posterior Mean (95% PI) |
No. of Estimates, No. of Studies |
|
| Total | 0.84 (0.48 to 1.20) | 11,9 | 0.92 (0.47 to 1.38) | 30,23 | 0.87 (0.55 to 1.18) | 41,32 |
| Cardiovascular disease | 0.85 (−0.66 to 2.39) | 5,4 | 1.09 (0.61 to 1.58) | 20,14 | 1.11 (0.68 to 1.53) | 25,18 |
| Respiratory | 0.65 (−1.84 to 3.21) | 4,4 | 0.45 (−0.74 to 1.65) | 19,13 | 0.47 (−0.51 to 1.47) | 23,17 |
Percent increase in mortality per 10-ppb increase in ozone at short-term lags (single-day lags of 0, 1, or 2 days or a 2-day average of lags 0 and 1 or lags 1 and 2.
Overall, we found that a 10-ppb increase in ozone in the few previous days (lags of 0, 1, or 2 days or a 2-day average of lags 0 and 1 or lags 1 and 2) is associated with a 0.87% increase in total mortality (95% posterior interval = 0.55% to 1.18%). Pooled effects were similar for studies within the United States and when studies outside the United States were included. When studies from all locations were considered, we found that the pooled effect for cardiovascular disease mortality is larger than for total mortality, whereas the pooled effect for respiratory mortality was lower (Table 2).
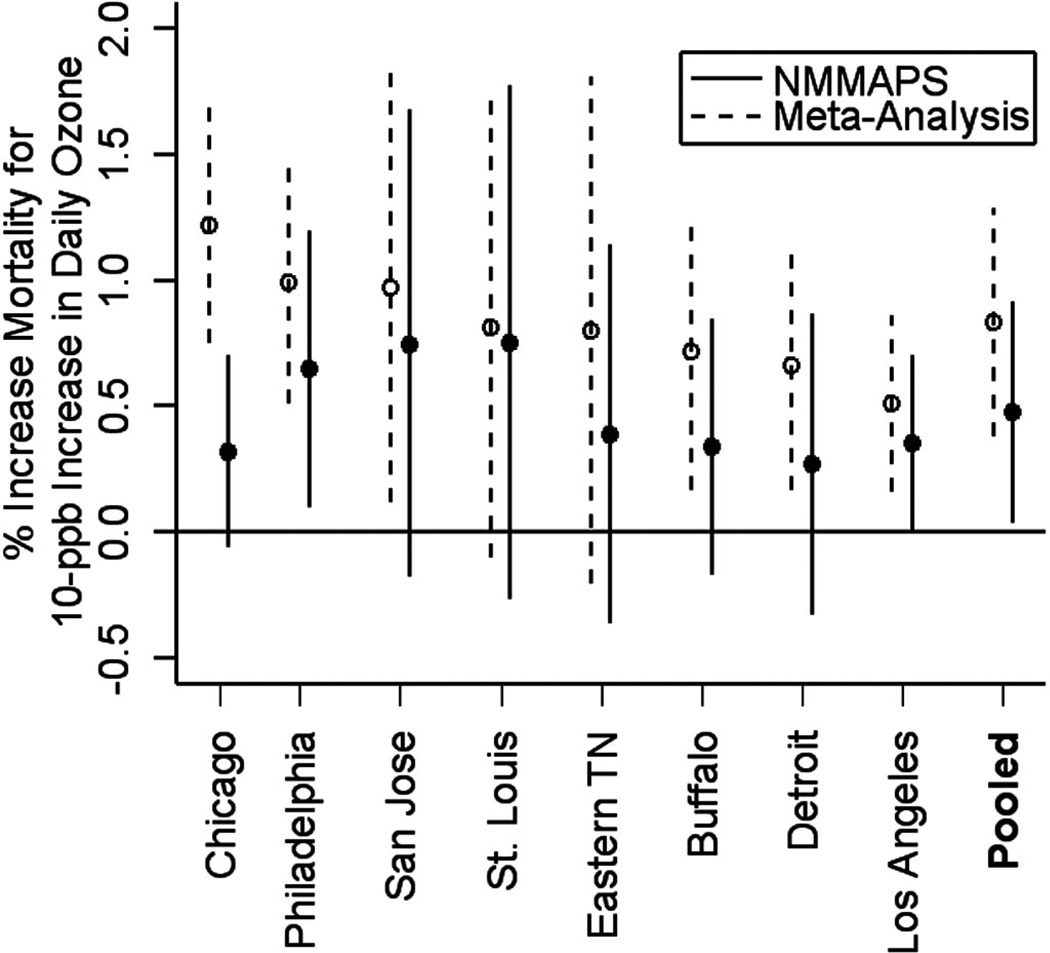
The pooled estimate for total mortality in the United States was based on 11 estimates from 9 studies in the following 9 communities: St. Louis; Kingston/Harriman, Tennessee; Santa Clara; Buffalo; Chicago; Philadelphia; Los Angeles; Detroit; and the Coachella Valley, California. Eight of these areas (all but the Coachella Valley) were included in NMMAPS ozone analysis.
We made 2 comparisons between the pooled effects obtained from the meta-analysis and from NMMAPS. First, we compared the pooled effects by including all the cities (9 from the meta-analysis and 95 from NMMAPS). Second, we restricted the comparison to the 8 cities that were included both in the meta-analysis and in NMMAPS.
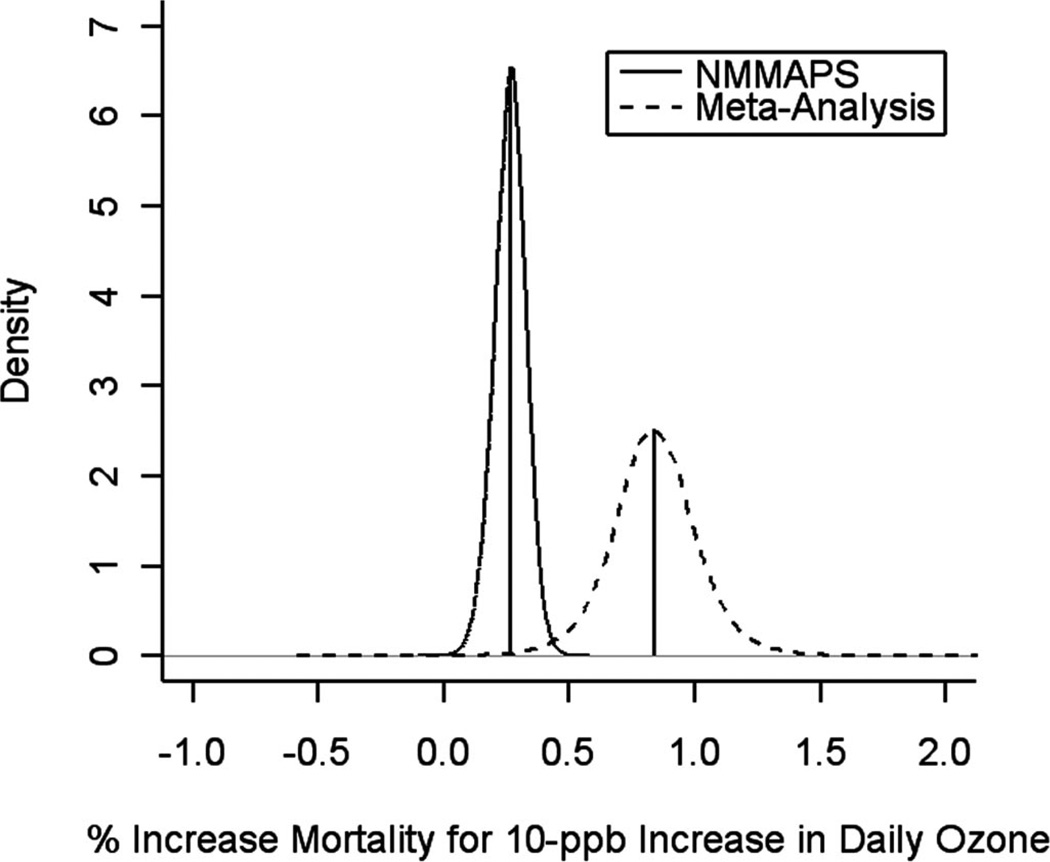
Figure 1 compares the marginal posterior distributions of the overall effect under the meta-analysis (based on 11 estimates from the 9 cities) and in NMMAPS (95 U.S. cities, all lag 0). When we combined information across the 95 cities, the national average effect of same-day ozone on mortality from NMMAPS was a 0.25% (95% posterior interval = 0.12% to 0.39%) increase in mortality for a 10-ppb increase in the same day’s ozone concentration. Figure 2 compares the marginal posterior distributions of the overall effect under the meta-analysis and in NMMAPS for the 8 cities common to both the approaches (8 U.S. cities, all lag 0). When we combined information across the 8 cities, the NMMAPS pooled effect of same-day ozone concentration was 0.48% (0.03% to 0.92%) as compared with the meta-analysis estimate of 0.83% (0.38% to 1.29%). In both cases (using the 8 cities or using all the estimates), the estimated pooled effects from NMMAPS were lower than estimates from the meta-analysis. This pattern is indicative of possible publication bias.
FIGURE 1.
Posterior distributions of pooled log-relative rates of all-cause mortality associated with 10-ppb increase in ozone in NMMAPS (95 cities) and for the meta-analysis of the United States (11 estimates).
FIGURE 2.
City-specific posterior means and 95% posterior intervals of the log-relative rate of mortality associated with 10-ppb increase in ozone for the 8 cities included in NMMAPS and in the meta-analysis.
The pooled effect from the meta-analysis for cardiovascular and respiratory mortality combined was slightly higher than the overall effect for total mortality. This pattern was also observed in the NMMAPS analyses.
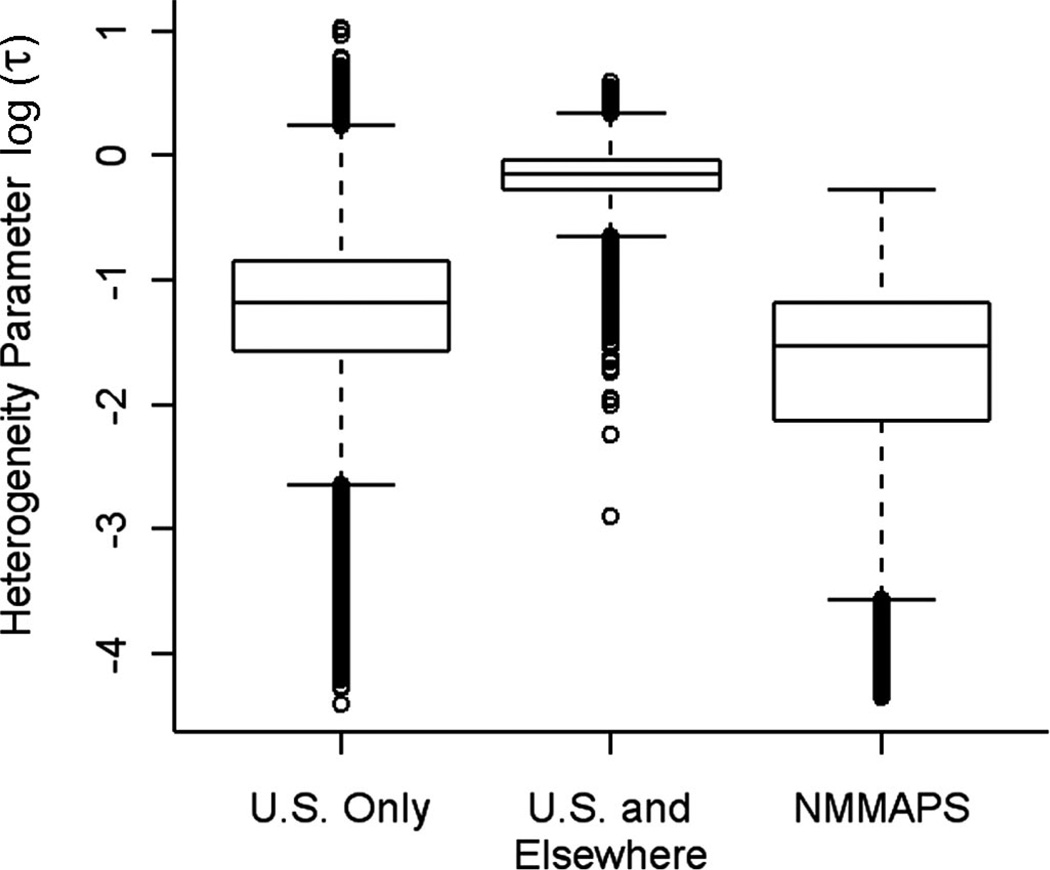
Figure 3 shows the posterior distribution of the heterogeneity parameter τ in log scale for total mortality and for the U.S. and non-U.S. studies combined. The city-specific effects included in the meta-analysis were more heterogeneous than estimates from NMMAPS. In the meta-analysis, there were several sources of heterogeneity in addition to potential differences between cities. These included differences in the specification of the statistical models, in the data quality, and the potential for publication bias, among other factors.
FIGURE 3.
Marginal posterior distribution of the log of heterogeneity parameter (τ) for: 1) meta-analysis of 11 U.S estimates; 2) meta-analysis of 41 U.S. and non-U.S. estimates; and 3) for 95 NMMAPS cities in Bell et al.38
In Table 3, we summarize the pooled estimates from the meta-analysis with and without adjustment for PM (either PM10 or PM2.5). In the time-series studies, the adjustment for PM was made by including the daily level of PM as a covariate in the Poisson regression model. Pooled effects were robust to the PM adjustment. These results are consistent with the recent NMMAPS analyses.38
TABLE 3.
Posterior Means and 95% Posterior Intervals of the Pooled Log-Relative Rates With and Without Adjustment for PM*
| U.S. Only |
U.S. and Non-U.S. |
|||
|---|---|---|---|---|
| PM Adjustment | Posterior Mean (95% PI) |
No. of Estimates, No. of Studies |
Posterior Mean (95% PI) |
No. of Estimates, No. of Studies |
| No | 0.84 (0.48 to 1.20) | 11,9 | 0.87 (0.55 to 1.18) | 41,32 |
| Yes | 0.74 (0.06 to 1.43) | 5,5 | 0.97 (−0.03 to 1.98) | 11,11 |
Percent increase in mortality per 10-ppb increase in ozone at short-term lags (single-day lags of 0, 1, or 2 days or a 2-day average of lags 0 and 1 or lags 1 and 2.
Table 4 shows posterior means and 95% posterior regions of the pooled effect for total mortality for lags 0, 1, and 2 from both the meta-analysis (using studies from the United States and elsewhere) and NMMAPS. For both analyses, the pooled effects were largest at lag 0 and smallest at lag 2.
TABLE 4.
Posterior Means and 95% Posterior Intervals of the Pooled Log-Relative Rates for Total Mortality at Various Single-Day Lags, Percent Increase in Mortality per 10-ppb Increase in Ozone
| Meta-Analysis |
NMMAPS |
||
|---|---|---|---|
| Lag (days) | Posterior Mean (95% PI) |
No. of Estimates, No. of Studies |
Posterior Mean (95% PI) |
| 0 | 0.81 (0.47 to 1.15) | 20,17 | 0.25 (0.12 to 0.39) |
| 1 | 0.58 (0.07 to 1.09) | 19,17 | 0.18 (0.06 to 0.30) |
| 2 | 0.27 (−0.05 to 0.60) | 10,9 | 0.14 (0.03 to 0.26) |
To further explore publication bias with respect to choice of lag, we calculated a pooled estimate for a variety of single-day lag times and compared the estimates for studies that provided results for only a single lag with those that provided multiple lags in the meta-analysis. More specifically, Table 5 compares pooled estimates obtained by combining studies that provided a single lag estimate (0 or 1) versus pooled estimates obtained by combining studies that reported estimates for multiple lags, including lags 0 or 1. The pooled effects from the studies that provided a single lag estimate were larger than those obtained from the studies that provided multiple estimates. This indicates that the lag with the highest effect is more likely to have been reported.
TABLE 5.
Posterior Means and 95% Posterior Intervals of the Pooled Log-Relative Rates by Reported Lags*
| Studies Providing Only a Single Lag |
Studies Providing Estimates for Multiple Lags |
|||
|---|---|---|---|---|
| Lag (days) | Posterior Mean (95% PI) |
No. of Estimates, No. of Studies |
Posterior Mean (95% PI) |
No. of Estimates, No. of Studies |
| 0 | 1.05 (0.42 to 1.69) | 11,9 | 0.66 (0.27 to 1.04) | 9,8 |
| 1 | 0.86 (−0.65 to 2.40) | 9,8 | 0.45 (0.08 to 0.82) | 10,9 |
Percent increase in mortality per 10-ppb increase in ozone.
Meta-analysis effect estimates are larger for the elderly (ie, 64 years and older or 65 and older). For this age category, a 10-ppb increase in daily ozone is associated with a 1.45% increase in total daily mortality (0.67% to 2.23%), including 10 estimates from 9 studies from both in and outside of the United States. This is higher than the estimate for all ages, at 0.87% (0.55% to 1.18%). The NMMAPS analyses found a slightly larger effect for the elderly.38
Table 6 shows the pooled effects for total mortality and for cardiovascular disease mortality, as obtained from studies that used the whole year’s data or that analyzed only data in the warmer time periods. Some time-series studies of ozone and mortality explored the relationship during a particular time of year such as May to October or only the summer. These warmer time periods reflect the peak ozone season, because the chemical reactions that form ozone are temperature-dependent.73 In the NMMAPS analysis, no appreciable difference was observed between the ozone and mortality relationship for the whole year and the association during May to October.
TABLE 6.
Posterior Means and 95% Posterior Intervals of the Pooled Log-Relative Rates for the Warm Time Periods and for the Whole Year*
| Yearly Data |
Warmer Time Periods |
|||
|---|---|---|---|---|
| Posterior Mean (95% PI) |
No. of Estimates, No. of Studies |
Posterior Mean (95% PI) |
No. of Estimates, No. of Studies |
|
| Total mortality | ||||
| U.S. | 0.84 (0.48 to 1.19) | 11,9 | 1.34 (−0.45 to 3.17) | 4,3 |
| U.S. and non-U.S. | 0.87 (0.55 to 1.18) | 41,32 | 1.50 (0.72 to 2.29) | 11,10 |
| Cardiovascular disease mortality | ||||
| U.S. and non-U.S. | 1.11 (0.68 to 1.53) | 25,18 | 2.45 (0.88 to 4.10) | 5,4 |
Percent increase in mortality per 10-ppb increase in ozone at short-term lags (single-day lags of 0, 1, or 2 days or a 2-day average of lags 0 and 1 or lags 1 and 2.
DISCUSSION
Both the meta-analysis and NMMAPS results provide strong evidence of an association between short-term exposure to ozone and mortality. Results from these 2 approaches have a consistent pattern of findings: larger effects for cardiovascular mortality (for the meta-analysis) and cardiovascular/respiratory mortality (for NMMAPS) than for total mortality; larger effects at lag 0 as compared with lags 1 or 2; and a lack of confounding by PM.
We found several indicators of publication bias in the reporting of time-series studies of ozone and mortality. The effect estimates for meta-analysis were much larger than those for the NMMAPS multicity analysis. In the meta-analysis, larger pooled effects were found among the studies that reported a single lag (either 0 or 1) as compared with those that reported multiple lags. This pattern suggests that the lag with the largest effect was more likely to be reported. A comparison of 21 time-series studies on PM10 and mortality with the NMMAPS analysis of 88 cities also provided evidence for publication bias.74 Evidence was also found in a recent meta-analysis of time-series and panel studies of ozone, particulate matter, and mortality.24 Therefore, although meta-analyses are very useful for combining information from different studies and investigating differences due to factors such as location or study design, they are likely to overestimate the true relationship between ozone and mortality.
Results from the new meta-analysis are consistent with earlier meta-analyses of ozone time-series studies, as shown in Table 7. Our meta-analysis indicates an association between short-term changes in ozone and mortality, with an estimated 0.87% increase in total mortality (95% posterior interval = 0.55% to 1.18%) for a 10-ppb increase in the daily average ozone level at lags of 0 (same day), 1 or 2 days or a 2-day average of lags 0 and 1 or lags 1 and 2. This result corresponds to approximately a 0.35% increase in mortality (0.22% to 0.47%) for a 10-ppb increase in the daily 1-hour maximum. To compare this estimate with other meta-analyses, all estimates must be based on the same measure of ozone concentration such as the daily average. Although the relationship between different ozone metrics varies by location, we used a relationship of 20:15:8 for the 1-hour maximum: 8-hour maximum:daily average, so that results are roughly comparable.20,21 For example, a 10-ppb increase in the daily average ozone concentration corresponds to approximately a 25-ppb increase in the daily 1-hour maximum concentration.
TABLE 7.
Comparison of Pooled Estimates From Other Meta-Analyses of Ozone and Mortality*
| Meta-Analysis Study | Percent Increase (95% Interval) |
|---|---|
| Thurston and Ito20 | 0.89 (0.56 to 1.22) |
| Thurston and Ito20 | 1.37 (0.78 to 1.96)* |
| Stieb et al 23 | 1.12 (0.32 to 1.92) |
| Levy et al 21 | 0.98 (0.59 to 1.38) |
| Anderson et al24 | 1.11 (0.55 to 1.67) |
| Present meta-analysis† | 0.87 (0.55 to 1.18) |
Included only studies that considered a nonlinear relationship between temperature and mortality.
Percent increase (95% posterior interval) in mortality per 10-ppb increase in daily average ozone at short-term lags (single-day lags of 0, 1, or 2 days or a 2-day average of lags 0 and 1 or lags 1 and 2.
A recent multicity study of 23 European cities found a 0.33% (95% CI = 0.17% to 0.52%) increase in daily mortality associated with a 10-µg/m3 increase in the average of the daily 1-hour maximum of the same and previous days during the warm season.26 These results are approximately comparable to a 1.63% (0.76% to 2.50%) increase in mortality for a 10-ppb increase in the daily average during the warm season, which is similar to the meta-analysis estimate of 1.62% (0.41% to 2.84%) for non-U.S. studies in the warm season based on 7 estimates from 7 studies. The European study found approximately a 2.22% (1.06% to 3.40%) increase in cardiovascular mortality for a 10-ppb increase in the daily average during the warm season, which is slightly lower than our meta-analysis estimates of 2.45% (0.88% to 4.10%) for cardiovascular mortality based on U.S. and non-U.S. studies combined for the warm season. We lacked a sufficient number of time-series studies to calculate a cardiovascular mortality estimate for the warm season for non-U.S. studies only.
The 1997 modification to the existing NAAQS was based largely on evidence of adverse respiratory effects that could be produced in laboratory experiments at ozone concentrations that were prevalent in many metropolitan areas of the United States. At that time, limited single-city time-series analyses indicated that ozone might also increase mortality on a short-term basis. The continued accumulation of results over the subsequent years shows consistent evidence of an effect of ozone on daily mortality counts (Table 7). As for the effect of particulate matter on mortality, a variety of mechanisms may be relevant, reflecting ozone’s potential to cause airway and pulmonary inflammation.
Although the meta-analysis results provide strong evidence for an effect of ozone on mortality, the comparison with results from NMMAPS provides evidence of publication bias. Such publication bias may have multiple explanations, from the choice of analytic strategies and pathways taken in model development to the tendency of investigators to submit findings that are “positive” and for journals to preferentially publish reports of statistically significant associations. Quantitative analyses of the public health impact of ozone based on single-city results or meta-analyses of such results would tend to overestimate the detrimental effect of ozone and the benefits of control. We recommend caution against using the results of single-city studies, whether individually or pooled, for impact assessment. Multicity approaches such as NMMAPS and APHENA offer a now-feasible alternative that is less subject to publication bias.
ACKNOWLEDGMENTS
We thank Roger D. Peng, Leah J. Welty, Joseph H. Abraham, Sorina Eftim, and Brian S. Caffo for their aid in coding the time-series studies.
Funding for Drs. Bell and Dominici was provided by the Environmental Protection Agency (EPA 3D-6867-NAEX). Funding for Drs. Dominici and Samet was provided by NIEHS ROI grant (ES012054-01) and by the NIEHS Center in Urban Environmental Health (P30-ES003819).
REFERENCES
- 1.US Environmental Protection Agency. National ambient air quality standards for particulate matter, final rule 62. Federal Register. 1997:38651. [Google Scholar]
- 2.The Ozone Report. EPA454/K-01-001. Research Triangle Park, NC: US EPA Office of Air Quality Planning and Standards; 2004. US Environmental Protection Agency. [Google Scholar]
- 3.Anderson HR, Ponce de Leon A, Bland JM, et al. Air pollution and daily mortality in London: 1987–92. BMJ. 1996;321:665–669. doi: 10.1136/bmj.312.7032.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cifuentes LA, Vega J, Köpfer K, et al. Effect of the fine fraction of particulate matter versus the coarse mass and other pollutants on daily mortality in Santiago, Chile. J. Air Waste Manage Assoc. 2000;50:1287–1298. doi: 10.1080/10473289.2000.10464167. [DOI] [PubMed] [Google Scholar]
- 5.Ha EH, Lee JT, Kim H, et al. Infant susceptibility of mortality to air pollution in Seoul, South Korea. Pediatrics. 2003;111:284–290. doi: 10.1542/peds.111.2.284. [DOI] [PubMed] [Google Scholar]
- 6.Hoek G, Brunekreef B, Verhoeff A, et al. Daily mortality and air pollution in the Netherlands. J Air Waste Manage Assoc. 2000;50:1380–1389. doi: 10.1080/10473289.2000.10464182. [DOI] [PubMed] [Google Scholar]
- 7.Kim SY, Lee JT, Hong YC, et al. Determining the threshold effect of ozone on daily mortality: an analysis of ozone and mortality in Seoul, Korea, 1995–1999. Environ Res. 2004;94:113–119. doi: 10.1016/j.envres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Kown HJ, Cho SH, Nyberg F, et al. Effects of ambient air pollution on daily mortality in a cohort of patients with congestive heart failure. Epidemiology. 2001;12:413–419. doi: 10.1097/00001648-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Moolgavkar SH. Air pollution and daily mortality in two US counties: season-specific analyses and exposure response relationships. Inhal Toxicol. 2003;15:877–907. doi: 10.1080/08958370390215767. [DOI] [PubMed] [Google Scholar]
- 10.Loomis DP, Borja-Aburto VH, Bangdiwala SI, et al. Ozone exposure and daily mortality in Mexico City: a time-series analysis. Res Rep Health Eff Inst. 1996;75:1–37. discussion 39–45. [PubMed] [Google Scholar]
- 11.Moolgavkar SH. Air pollution and mortality in three US counties. Environ Health Perspect. 2000;108:777–784. doi: 10.1289/ehp.00108777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson R, Denison L, Petroeschevsky A, et al. Effects of ambient particle pollution on daily mortality in Melbourne, 1991–1996. J Expo Anal Environ Epidemiol. 2000;10:448–496. doi: 10.1038/sj.jea.7500137. [DOI] [PubMed] [Google Scholar]
- 13.O’Neill MS, Loomis D, Borja-Aburto VH. Ozone, area social conditions, and mortality in Mexico City. Environ Res. 2004;94:234–242. doi: 10.1016/j.envres.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Anderson HR, Bremner SA, Atkinson RW, et al. Particulate matter and daily mortality and hospital admissions in the west midlands conurbation of the United Kingdom: associations with fine and coarse particles, black smoke and sulfates. Occup Environ Med. 2001;58:504–510. doi: 10.1136/oem.58.8.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fairley D. Revised Analyses of Time-Series Studies of Air Pollution and Health. Cambridge: Health Effects Institute; 2003. Mortality and air pollution for Santa Clara County, California, 1989–1996; pp. 97–106. [Google Scholar]
- 16.Hong YC, Leem JH, Ha EH. Air pollution and daily mortality in Inchon, Korea. J Korean Med Sci. 1999;14:239–244. doi: 10.3346/jkms.1999.14.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klemm RJ, Mason RM. Aerosol Research and Inhalation Epidemiological Study (ARIES): air quality and daily mortality statistical modeling - interim results. J Air Waste Manage Assoc. 2000;50:1433–1439. doi: 10.1080/10473289.2000.10464188. [DOI] [PubMed] [Google Scholar]
- 18.Ostro BD, Broadwin R, Lipsett MJ. Coarse and fine particles and daily mortality in the Coachella Valley, California: a follow-up study. J Expo Anal Environ Epidemiol. 2000;10:412–419. doi: 10.1038/sj.jea.7500094. [DOI] [PubMed] [Google Scholar]
- 19.Roemer WH, vanWijnen JH. Daily mortality and air pollution along busy streets in Amsterdam, 1987–1998. Epidemiology. 2001;12:649–653. doi: 10.1097/00001648-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Thurston GD, Ito K. Epidemiological studies of acute ozone exposures and mortality. J Expo Anal Environ Epidemiol. 2001;11:286–294. doi: 10.1038/sj.jea.7500169. [DOI] [PubMed] [Google Scholar]
- 21.Levy JI, Carrothers TJ, Tuomisto JT, et al. Assessing the public health benefits of reduced ozone concentrations. Environ Health Perspect. 2001;109:1215–1226. doi: 10.1289/ehp.011091215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manage Assoc. 2002;52:470–484. doi: 10.1080/10473289.2002.10470794. [DOI] [PubMed] [Google Scholar]
- 23.Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: update in relation to the use of generalized additive models. J Air Waste Manage Assoc. 2003;53:258–261. doi: 10.1080/10473289.2003.10466149. [DOI] [PubMed] [Google Scholar]
- 24.Anderson HR, Atkinson RW, Peacock JL, et al. Meta-Analysis of Time-Series Studies and Panel Studies of Particulate Matter (PM) and Ozone (O3). Report of a WHO Task Group. Copenhagen: World Health Organization; 2004. [Google Scholar]
- 25.Touloumi G, Katsouyanni K, Zmirou D, et al. Short-term effects of ambient oxidant exposure on mortality: a combined analysis within the APHEA project. Air Pollution and Health: a European Approach. Am J Epidemiol. 1997;146:177–185. doi: 10.1093/oxfordjournals.aje.a009249. [DOI] [PubMed] [Google Scholar]
- 26.Gryparis A, Forsberg B, Katsouyanni K, et al. Acute effects of ozone on mortality from the ‘Air Pollution and Health: A European Approach’ project. Am J Respir Crit Care Med. 2004;170:1080–1087. doi: 10.1164/rccm.200403-333OC. [DOI] [PubMed] [Google Scholar]
- 27.Le Tertre A, Quenel P, Eilstein D, et al. Short-term effects of air pollution on mortality in nine French cities: a quantitative summary. Arch Environ Health. 2002;57:311–319. doi: 10.1080/00039890209601414. [DOI] [PubMed] [Google Scholar]
- 28.Health Effects Institute. Revised Analyses of Time-Series Studies of Air Pollution and Health: Revised Analyses of the National Morbidity, Mortality, and Air Pollution Study, Part II, Revised Analyses of Selected Time-Series Studies. Cambridge, MA: Health Effects Institute; 2003. [Google Scholar]
- 29.Saez M, Ballester F, Barcelo MA, et al. A combined analysis of the short-term effects of photochemical air pollutants on mortality within the EMECAM project. Environ Health Perspect. 2002;110:221–218. doi: 10.1289/ehp.02110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JT, Kim H, Hong YC, et al. Air pollution and daily mortality in seven major cities of Korea, 1991–1997. Environ Res. 2000;84:247–254. doi: 10.1006/enrs.2000.4096. [DOI] [PubMed] [Google Scholar]
- 31.Zmirou D, Schwartz J, Saez M, et al. Time-series analysis of air pollution and cause-specific mortality. Epidemiology. 1998;9:495–503. [PubMed] [Google Scholar]
- 32.Samet JM, Dominici F, Curriero FC, et al. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 33.Samet JM, Dominici F, Zeger SL, et al. The National Morbidity, Mortality, and Air Pollution Study Part I: Methods and Methodologic Issues. Cambridge, MA: Health Effects Institute; 2000. [PubMed] [Google Scholar]
- 34.Samet JM, Zeger SL, Dominici F, et al. The National Morbidity, Mortality, and Air Pollution Study Part II: Morbidity and Mortality from Air Pollution in the United States. Cambridge, MA: Health Effects Institute; 2000. [PubMed] [Google Scholar]
- 35.Dominici F, McDermott A, Zeger SL, et al. National maps of the effects of particulate matter on mortality: exploring geographical variation. Environ Health Perspect. 2003;111:39–43. doi: 10.1289/ehp.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dominici F, Daniels M, Zeger SL, et al. Air pollution and mortality: estimating regional and national dose–response relationships. J Am Stat Assoc. 2002;97:100–111. [Google Scholar]
- 37.Dominici F, Samet J, Zeger S. Combining evidence on air pollution and daily mortality from the largest 20 US cities: a hierarchical modeling strategy. J R Stat Soc [Ser A] 2000;163:263–302. [Google Scholar]
- 38.Bell ML, McDermott A, Zeger SL, et al. Ozone and mortality in 95 US urban communities, 1987–2000. JAMA. 2004;292:2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominici F, McDermott A, Zeger SL, et al. On the use of generalized additive models in time-series studies of air pollution and health. Am J Epidemiol. 2002;156:193–203. doi: 10.1093/aje/kwf062. [DOI] [PubMed] [Google Scholar]
- 40.Lindley DV, Smith AFM. Bayes estimates for the linear model. J R Stat Soc. 1972;34:1–41. [Google Scholar]
- 41.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 42.DuMouchel W. Bayesian Metaanalysis. New York: Dekker; 1990. [Google Scholar]
- 43.DuMouchel WH, Harris JE. Bayes methods for combining the results of cancer studies in humans and other species. J Am Stat Assoc. 1983;78:293–315. [Google Scholar]
- 44.Gelman A, Carlin J, Stern H, et al. Bayesian Data Analysis. Chapman and Hall; 1995. [Google Scholar]
- 45.Spiegelhalter D, Thomas A, Best N, et al. Winbugs, version 1.4. 2003. [Google Scholar]
- 46.Bernardo JM, Smith AFM. Bayesian Theory. New York: John Wiley & Sons; 2000. [Google Scholar]
- 47.Bremner SA, Anderson HR, Atkinson RW, et al. Short term associations between outdoor air pollution and mortality in London 1992–4. Occup Environ Med. 1999;56:237–244. doi: 10.1136/oem.56.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borja-Aburto VH, Loomis DP, Bangdiwala SI, et al. Ozone, suspended particulates, and daily mortality in Mexico City. Am J Epidemiol. 1997;145:258–268. doi: 10.1093/oxfordjournals.aje.a009099. [DOI] [PubMed] [Google Scholar]
- 49.Borja-Aburto VH, Castillejos M, Gold DR, et al. Mortality and ambient fine particles in southwest Mexico City, 1993–1995. Environ Health Perspect. 1998;106:849–855. doi: 10.1289/ehp.106-1533229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnett RT, Cakmak S, Raizenne ME, et al. The association between ambient carbon monoxide levels and daily mortality in Toronto, Canada. J Air Waste Manage Assoc. 1998;48:689–700. doi: 10.1080/10473289.1998.10463718. [DOI] [PubMed] [Google Scholar]
- 51.Dab W, Medina S, Quénel P, et al. Short-term respiratory health effects of ambient air pollution: results of the APHEA project in Paris. J Epidemiol Community Health. 1996;50:S42–S46. doi: 10.1136/jech.50.suppl_1.s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dockery DW, Schwartz J, Spengler JD. Air pollution and daily mortality: associations with particulates and acid aerosols. Environ Res. 1992;59:362–373. doi: 10.1016/s0013-9351(05)80042-8. [DOI] [PubMed] [Google Scholar]
- 53.Goldberg MS, Burnett RT, Brook J, et al. Associations between daily cause-specific mortality and concentrations of ground-level ozone in Montreal, Quebec. Am J Epidemiol. 2001;154:817–826. doi: 10.1093/aje/154.9.817. [DOI] [PubMed] [Google Scholar]
- 54.Gwynn RC, Burnett RT, Thurston GD. A time-series analysis of acidic particulate matter and daily mortality and morbidity in the Buffalo, New York, region. Environ Health Perspect. 2000;108:125–133. doi: 10.1289/ehp.00108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoek G. Revised Analysis of Time-Series Studies of Air Pollution and Health. Cambridge, MA: Health Effects Institute; 2003. Daily mortality and air pollution in The Netherlands; pp. 133–141. [Google Scholar]
- 56.Hong YC, Lee JT, Kim H, et al. Effects of air pollutants on acute stroke mortality. Environ Health Perspect. 2002;110:187–191. doi: 10.1289/ehp.02110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito K, Thurston GD. Daily PM10/mortality associations: an investigation of at-risk subpopulations. J Expo Anal Environ Epidemiol. 1996;6:79–95. [PubMed] [Google Scholar]
- 58.Kelsall JE, Samet JM, Zeger SL, et al. Air pollution and mortality in Philadelphia , 1974–1988. Am J Epidemiol. 1997;146:750–762. doi: 10.1093/oxfordjournals.aje.a009351. [DOI] [PubMed] [Google Scholar]
- 59.Kinney PL, Ito K, Thurston GD. A sensitivity analysis of mortality/PM10 associations in Los Angeles. Inhal Toxicol. 1995;7:59–69. [Google Scholar]
- 60.Lee JT, Shin D, Chung Y. Air pollution and daily mortality in Seoul and Ulsan, Korea. Environ Health Perspect. 1999;107:149–154. doi: 10.1289/ehp.99107149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lippmann M, Ito K, Nadas A, et al. Association of particulate matter components with daily mortality and morbidity in urban populations. Res Rep Health Eff Inst. 2000;95:5–72. discussion 73–82. [PubMed] [Google Scholar]
- 62.Zmirou D, Barumandzadeh T, Balducci F, et al. Short term effects of air pollution on mortality in the city of Lyon, France, 1985–90. J Epidemiol Community Health. 1996;50:S30–S35. doi: 10.1136/jech.50.suppl_1.s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moolgavkar SH, Luebeck EG, Hall TA, et al. Air pollution and daily mortality in Philadelphia. Epidemiology. 1995;6:476–484. doi: 10.1097/00001648-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Morgan G, Corbett S, Wlodarczyk J, et al. Air pollution and daily mortality in Sydney, Australia, 1989 through 1993. Am J Public Health. 1998;88:759–764. doi: 10.2105/ajph.88.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ostro B. Fine particulate air pollution and mortality in two Southern California counties. Environ Res. 1995;70:98–104. doi: 10.1006/enrs.1995.1053. [DOI] [PubMed] [Google Scholar]
- 66.Ostro BD, Sanchez JM, Aranda C, et al. Air pollution and mortality: results from a study of Santiago, Chile. J Expo Anal Environ Epidemiol. 1996;6:97–114. [PubMed] [Google Scholar]
- 67.Ostro BD, Hurley S, Lipsett MJ. Air pollution and daily mortality in the Coachella Valley, California: a study of PM10 dominated by coarse particles. Environ Res. 1999;81:231–238. doi: 10.1006/enrs.1999.3978. [DOI] [PubMed] [Google Scholar]
- 68.Prescott GJ, Cohen GR, Elton RA, et al. Urban air pollution and cardiopulmonary ill health: a 14.5 year time series study. Occup Environ Med. 1998;55:697–704. doi: 10.1136/oem.55.10.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sunyer J, Castellsagué J, Sáez M, et al. Air pollution and mortality in Barcelona. J Epidemiol Community Health. 1996;50:S76–S80. doi: 10.1136/jech.50.suppl_1.s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verhoeff AP, Hoek G, Schwartz J, et al. Air pollution and daily mortality in Amsterdam. Epidemiology. 1996;7:225–230. doi: 10.1097/00001648-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 71.Wietlisbach V, Pope CA, Ackermann-Liebrich U. Air pollution and daily mortality in three Swiss urban areas. Soz Praventivmed. 1996;41:107–115. doi: 10.1007/BF01323089. [DOI] [PubMed] [Google Scholar]
- 72.Ramsay TO, Burnett RT, Krewski D. The effect of concurvity in generalized additive models linking mortality to ambient particulate matter. Epidemiology. 2003;14:18–23. doi: 10.1097/00001648-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 73.Seinfeld JH, Pandis SN. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. New York: John Wiley & Sons, Inc; 1998. [Google Scholar]
- 74.Smith R, Guttorp P, Sheppard L, et al. Comments on the Criteria Document for Particulate Matter Air Pollution. NRCSE Technical Report Series No. 066. 2001