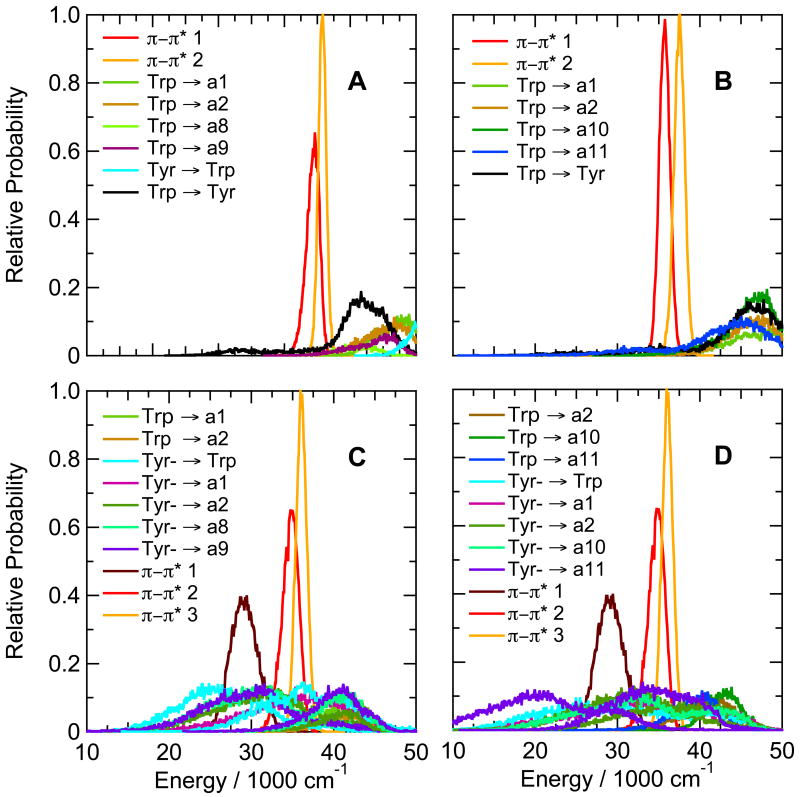
Figure 7.
Calculated distributions of excitation energies of excited singlet π–π* states and CT states of hairpin peptides with Trp at position 1 and Tyr at either position 8 (Ac-WVTIpGKYIFTG-NH2, A, C) or position 10 (Ac-WVTIpGKAIYTG-NH2, B, D), with the Tyr side chain either neutral (A, B) or ionized (C, D). The energy distributions were averaged over twenty 0.5-ns trajectories in the first excited singlet state. Backbone amide groups are designated as in Figure 3; states involving electron transfer between the indole ring of Trp and the phenol ring of Tyr are designated “Trp” → “Tyr”, “Tyr → Trp” and (for ionized Tyr) “Tyr- → Trp”. When the Tyr is ionized, the first excited π–π* state (π–π*1, dark brown) is localized mainly on the phenolate ring; the second and third π–π* states (red and orange), mainly on the indole ring of Trp1. When the Tyr is neutral (panels A and B), π–π*1 and π–π*2 (red and orange) are localized mainly on Trp1, and the first excited singlet state of the phenol group is at higher energies (not shown). CT states with two different configurations are shown for electron transfer from ionized Tyr to amides a1, a2, a8 and a9 in C, and to amides a1, a2, a10 and a11 in D.