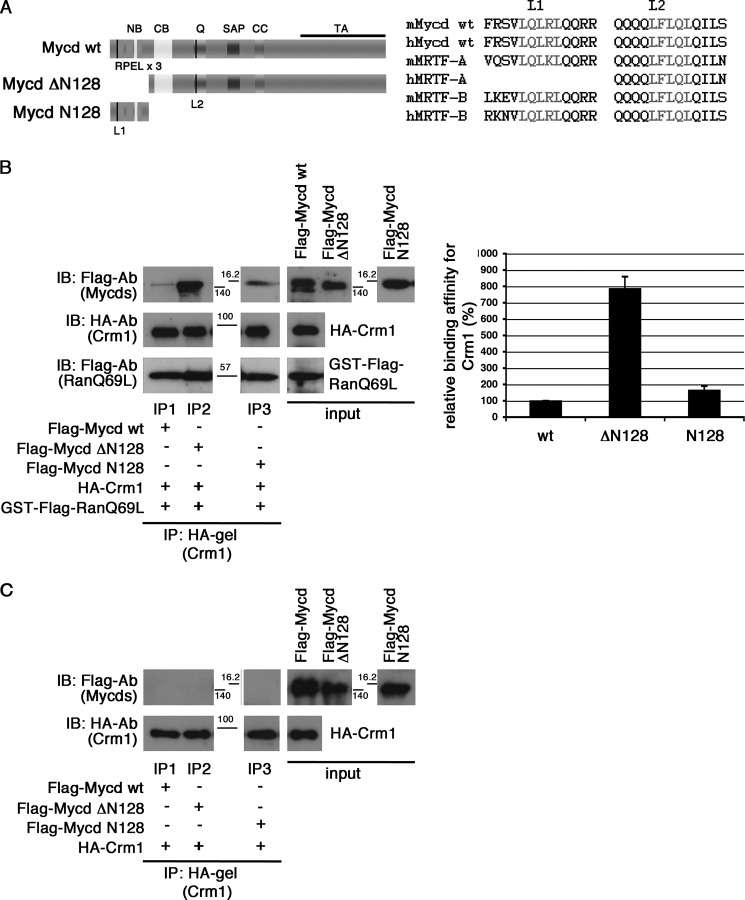
FIGURE 1.
Interaction between CRM1 and Mycd WT. A, left, schematic representation of Mycd WT and truncated Mycd isoforms. The two Leu-rich sequences are indicated by black vertical lines: L1 in the first RPEL motif and L2 in the Gln-rich domain (Q). NB, N-terminal basic domain; CC, coiled-coil domain; TA, transactivation domain. Right, sequences of L1 and L2 (gray letters) in Mycd WT and MRTF-A/B orthologs from different species (mouse (m) and human (h)). B and C, interaction between CRM1 and Mycd WT or each truncated Mycd. Mixtures of HA-tagged CRM1 and each indicated FLAG-tagged Mycd with (B) or without (C) GTP-bound GST-FLAG-RanQ69L were immunoprecipitated with a control gel or anti-HA affinity gel, and the immunoprecipitates thus obtained were analyzed by IB using the indicated antibodies (left). The positions of molecular mass markers are between the panels in kilodaltons. Control experiments using the control gel did not show any significant signals on immunoblots (data not shown). Quantification of the IP analysis is shown (B, right). The respective IP/IB signal intensities were quantified as described under “Experimental Procedures.” The percentages indicate relative binding affinities for CRM1 normalized by the affinity of Mycd WT, which was set at 100%. Results are means ± S.E. of three independent experiments (error bars).