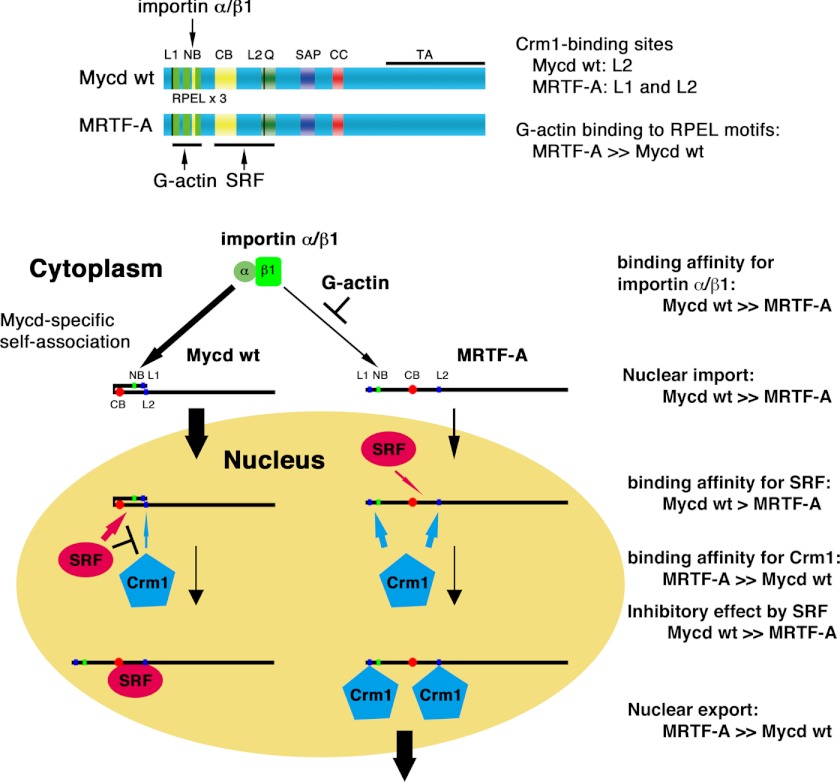
FIGURE 12.
Summary of differential regulation of subcellular localization between Mycd and MRTF-A. Our current findings combined with our previous ones are schematically represented. The nuclear accumulation of Mycd is determined by its high binding affinity for the importin α/β1 heterodimer and its molecular design for exclusion from CRM1-mediated nuclear export (autoinhibition by self-association and protection by SRF binding). In contrast, the binding affinity of MRTF-A for the importin α/β1 heterodimer is lower than that of Mycd, and this interaction is competitively inhibited by G-actin. Thus, MRTF-A is less likely to be imported to the nucleus. In contrast, MRTF-A is much more likely to be exported from the nucleus by CRM1 because of its high binding affinity for CRM1, its low binding affinity for SRF, and absence of autoinhibition. These differences may cause a distinct subcellular localization of Mycd and MRTF-A. NB, N-terminal basic domain; Q, Gln-rich domain; CC, coiled-coil domain; TA, transactivation domain.