FIGURE 5.
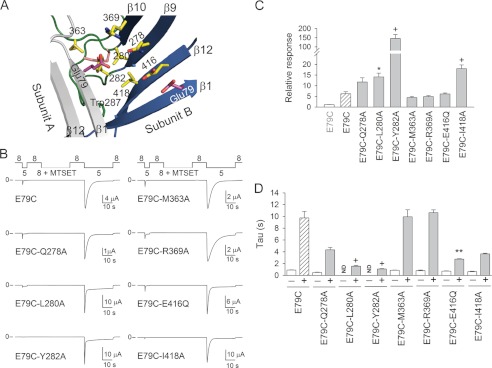
Ala substitutions in the palm and thumb domains alter E79C proton gating. A, structural model of ASIC1 in the desensitized state illustrating residues neighboring Glu-79. Subunit A is shown in gray, and subunit B is multicolored. B, representative recordings of experiments performed with oocytes expressing E79C (control) and double mutant channels exposed to MTSET at pH 8.0. Whole-cell currents were evoked by a change in extracellular pH from 8.0 to 5.0. Note the deficient response of non-modified E79C/Y282A channels to extracellular acidification. C, relative response to extracellular acidification of covalently modified E79C and double mutant channels. The relative response represents the ratio of the pH-elicited peak current following MTSET treatment to the pH-elicited peak current before treatment (n = 10–26). Statistically significant differences between covalently modified double mutant channels and covalently modified E79C channels are indicated: *, p < 0.05; +, p < 0.001 (Kruskal-Wallis test followed by Dunn's multiple comparison test). D, time constants of desensitization of ASIC1a mutant channels. Whole-cell currents were fitted to a single exponential function as described under “Experimental Procedures.” Statistically significant differences between covalently modified double mutant channels and covalently modified E79C channels are indicated: **, p < 0.01; +, p < 0.001 (Kruskal-Wallis test followed by Dunn's multiple comparison test). ND, not determined.
