FIGURE 2.
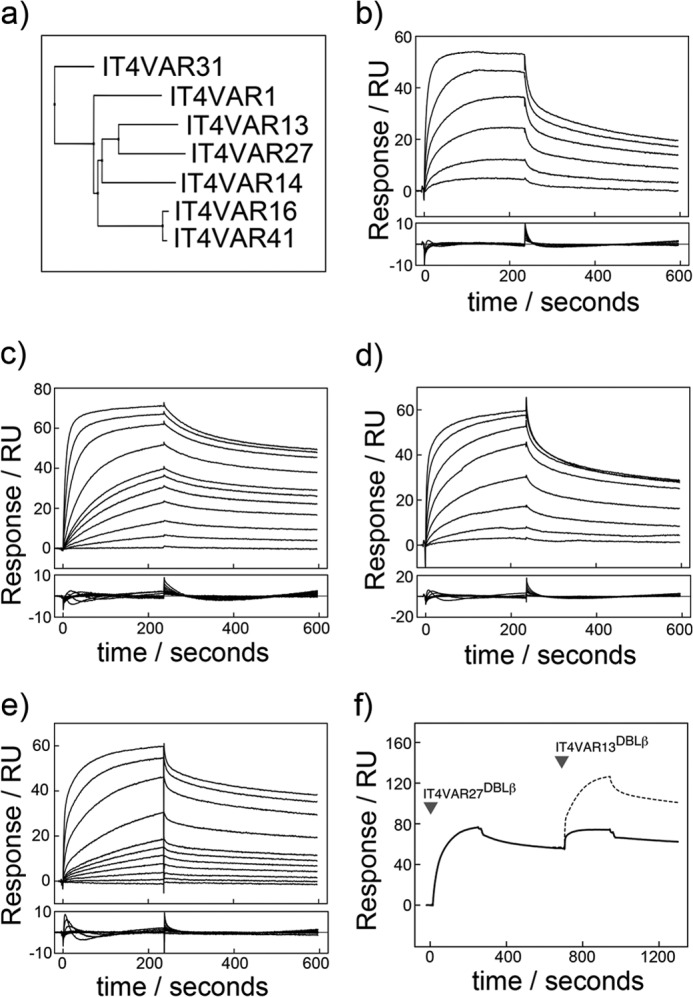
Characterization by SPR of the interactions of ICAM-1 with multiple DBLβ domains from the P. falciparum IT4 isolate. a, shown is a phylogenetic tree of seven PfEMP1 DBLβ domains known to bind ICAM-1 from the P. falciparum IT4 isolate. Shown are sensorgrams (upper panel) with resulting residuals when fit to a one-site kinetic model (lower panel). Shown are SPR sensorgrams (upper panels) with fitting residuals (lower panels) for the binding of IT4VAR16DBLβ (50, 100, 250, 500, 1000, and 2000 nm) (b), IT4VAR27DBLβ (1, 5, 10, 20, 30, 40, 50, 100, 250, 500, and 1000 nm) (c), IT4VAR31DBLβ (0.05, 0.1, 0.25, 1, 2, 5, and 10 μm) (d), and IT4VAR41DBLβ (1, 5, 10, 20, 30, 40, 50, 100, 250, 500, and 1000 nm) (e) to ICAM-1D1D2-Fc with an association phase of 4 min and a dissociation phase of 6 min at a flow rate of 30 μl min−1. f, DBLβ domains from IT4VAR13 and IT4VAR27 recognize ICAM-1 with overlapping binding sites. Expected binding levels assuming IT4VAR13DBLβ bound to ICAM-1 in a mode independent and unaffected by the binding of IT4VAR27DBLβ are shown with a dashed line. Actual binding levels shown by a solid line.
