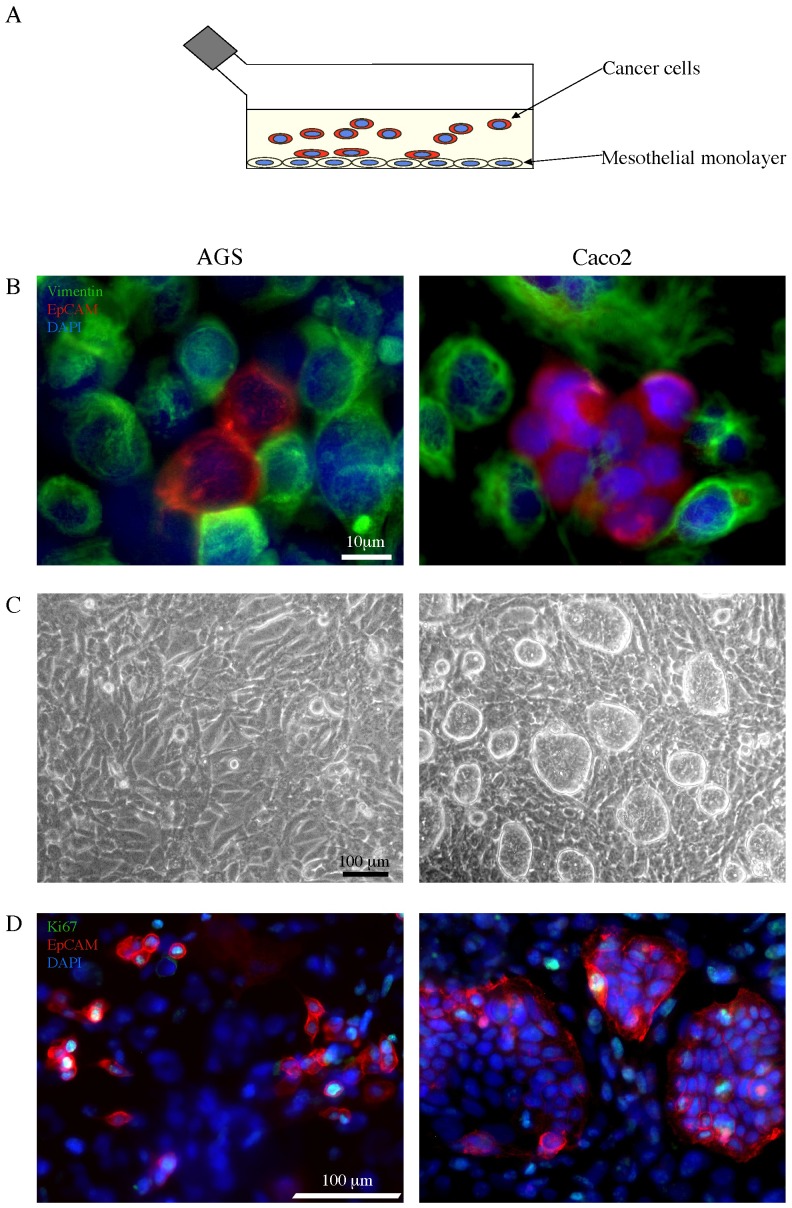
Figure 1. Co-culture in vitro test for the adhesion of cancer cell lines to mesothelial monolayer.
A) Schematic drawing of the co-culture system and the adhesion test used throughout the study: cancer cells are seeded on a mesothelial monolayer to evaluate cell adhesion. B) MeT-5A mesothelial cell line was grown at confluence and AGS or Caco2 cells were seeded on the mesothelial monolayer in co-culture (25.000 cells/cm2). After 24 hours from seeding, the co-culture was fixed, permeabilized and stained with a primary antibody directed against vimentin, followed by a secondary Ab labeled with the FITC fluorocrome (green) to identify the mesothelial cells. Double immunofluorescence with α-EpCAM PE antibody (red) was performed to recognize the cancer epithelial cells. Cellular nuclei were stained with DAPI (blue). The immunofluorescence analysis reveals the different cell types in our co-culture model. The signal corresponding to vimentin in the cell monolayer is compatible with that of intermediate filaments, as perinuclear cytoplasmic bundles, while the EpCAM staining is associated with the plasma membrane of the cancer cells. Both AGS and Caco2 cells appear either in small clusters or isolated and strictly adherent to the mesothelial cells. Bar: 10 µm. C) Phase contrast microscopy used to verify the integrity of mesothelium monolayer. After 48 hours from seeding, the adherent Caco2 cells display a pattern of growing in compact islands, while the AGS adhering cells show a more flattened shape and an isolated pattern of growth. Bar: 100 µm. D) Proliferation assay performed by immunofluorescence staining with a primary anti-Ki67 antibody, which identifies cycling cells, followed by a secondary FITC-labeled Ab (green). The tumor cells were labeled with the anti-EpCAM PE Ab as above. After 48 hours from seeding, the distribution of the cancer cells positive for the Ki67 nuclear signal reveals a different behavior of tumor growth: differently from the isolated AGS cells, the Ki67+ Caco2 cells are located at the periphery of the islands. Bar: 100 µm.