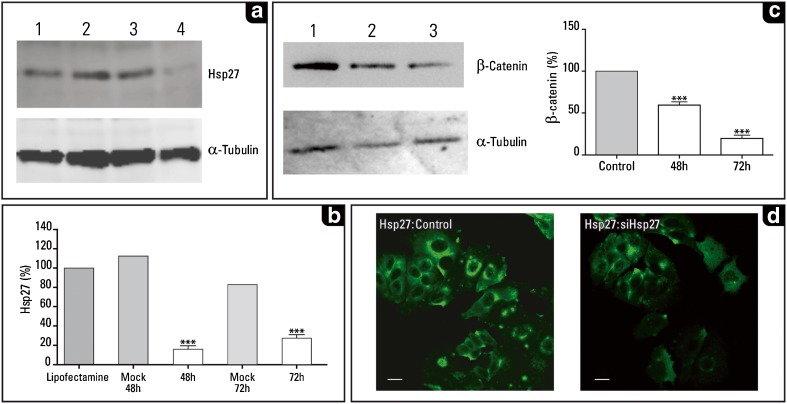
Fig. 1.
Downregulation of Hsp27 and its effect on β-catenin expression in MCF-7 cells. a Hsp27 levels as revealed in the western blot analysis (performed as described previously; Fanelli et al. 2008). The antibodies used were: a mouse monoclonal antibody against Hsp27 (1:1,000; cat. # SPA-800, Stressgen Biotech. Corp., Victoria, Canada), b mouse monoclonal antibody against α-tubulin (1:16,000; Sigma Chem. Co., St. Louis, MO, USA), and c mouse monoclonal antibody anti-β-catenin (1:500; cat. # 18-0226; Zymed, Carlsbad, CA, USA). 1 Control untreated cells, 2 control cells treated with Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA), 3 control cells transfected with the empty vector for 72 h, 4 note the decreased expression of Hsp27 in the siHsp27 transfected cells (72 h after transfection). The immunoblots images were capture using LAS-4000 imaging system (Fujifilm Life Sc., USA). b Graph showing a significant depletion of Hsp27 at 48 h (immunoblot not shown) and 72 h after siHsp27 transfection. Mock 48 and 72 h were MCF-7 cells transfected with empty vector and analyzed at 48 and 72 h after transfection, respectively. The evaluation of the immunoblots was performed using NIH image V1,62 program (NIH, Bethesda, MD, USA). The data were analyzed with the Prism computer program (Graph Pad Software, San Diego, CA, USA); data shown are means ± standard errors of the mean of three independent experiments. Statistical significance was assessed by column analyses with one-way ANOVA, the level of significance was set at p < 0.05. c Immunoblot showing the significant decrease of β-catenin. 1 Control untreated MCF-7 cells, 2 and 3 cells transfected with siHSP27 (48 and 72 h after transfection; p < 0.05). d Immunofluorescence of MCF-7 cells showing: a the basal Hsp27 levels (left panel, control cells treated with Lipofectamine™ 2000), and b the decreased Hsp27 levels after 72 h of siHsp27 transfection (right panel). Bar 10 μm. The MCF-7 human breast cancer cell line was kindly provided by Dr. MC Abba [Centro de Investigaciones Inmunológicas Básicas y Aplicadas (CINIBA), Universidad Nacional de La Plata, Argentina]. The cells were routinely cultured in Dulbecco’s Modified Eagle Medium (GIBCO, Invitrogen Corp, Argentina) supplemented with 10 % fetal calf serum (GIBCO) and 100 IU/ml penicillin and 100 μg/ml streptomycin (GIBCO) at 37 °C in an incubator with 5 % CO2 and 100 % humidity. Subconfluent cells were split twice a week at a ratio of 1:20. For knockdown of Hsp27 expression, transient transfections were done with 2 μg/ml pSIREN-RetroQ empty vector (Mock-transfection control) and shHsp27-pSIREN-RetroQ vector for 5h using LipofectamineTM 2000 (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s recommendations. The shHsp27-pSIREN-RetroQ vector was gently provided by Dr. MY Sherman (Boston University Medical School, Boston, MA, USA; O’Callaghan-Sunol et al. 2007). The vector contained the sequence of human Hsp27 (accession number NM 001540) as target for RNA interference: shHsp27 (start 701): ATCCGATGAGACTGCCGCCAA. The transfection efficiency was evaluated in each experiment using pSIREN-DNR-DsRed-Express (a gift from Dr. MY Sherman). After the start of transfection at 48 and 72 h, cells were washed twice in ice-cold PBS, lysed with cell lysis buffer (triton-x buffer with protease inhibitors) and stored at −80 °C for immunoblotting analysis. Immunofluorescence staining: MCF-7 cells were fixed with 2 % paraformaldehyde in PBS for 10 min at 37 °C, washed with PBS and blocked with 50 mM NH4Cl in PBS. Then the cells were permeabilized with 0.05 % saponin in PBS containing 0.5 % BSA, and incubated with primary antibody against Hsp27 (1:100). After washing, cells were incubated with secondary antibody conjugated with FITC (1:500; Jackson ImmunoResearch Laboratories Incorporated, West Grove, PA, USA). MCF-7 cells were mounted with Mowiol (Sigma-Aldrich, Argentina) and examined by confocal microscopy using an FV1000 Olympus Confocal Microscope and FV 10-ASW 1.7 software (Olympus, Japan). Images were processed using ImageJ software