Abstract
Oxidative stress and cellular injury have been implicated in induction of HSP72 by alcohol. We investigated the association between HSP72 induction and oxidative stress in mouse tissues following short-term administration of high doses of alcohol and caffeine alone or in combination. Adult male C57BL/6J mice were gavaged with vehicle, alcohol (∼1.7 g/kg/day), caffeine (∼44 mg/kg/day), or alcohol plus caffeine once daily for ten consecutive days. Upon completion of the treatments, tissues were collected for structural and biochemical analyses. Alcohol alone caused mild to moderate lesions in heart, liver, and gastrocnemius muscle. Similar structural changes were observed following administration of alcohol and caffeine combined. Alcohol administration also led to decreased glutathione levels in all three tissues and reduced plasma superoxide dismutase capacity. In contrast, alcohol and caffeine in combination reduced glutathione levels only in liver and gastrocnemius muscle and had no effect on plasma superoxide dismutase. Significant elevations in HSP72 protein and mRNA and in HSF1 protein levels were noted only in liver by alcohol alone or in combination with caffeine. No significant changes in morphology and HSP72 were detected in any tissues tested following administration of caffeine alone. These results suggest that a redox mechanism is involved in the structural impairment caused by short-term high-dose alcohol. Oxidative tissue injury by alcohol may not be associated with tissue HSP72 induction. Induction of HSP72 in liver by alcohol is mediated at both the transcriptional and translational levels.
Keywords: Ethanol, Coffee, Cytokines, Superoxide dismutase, 8-Isoprotane
Introduction
One of the leading preventable causes of death in the USA is related to alcohol consumption (Mokdad et al. 2004). Chronic consumption of alcohol by humans is commonly associated with multiple organ dysfunctions such as liver lesions, cardiomyopathy, and skeletal muscle myopathy (Fromenty and Pessayre 1995; Lieber 1988). Oxidative stress has been suggested to be one mechanism in the pathogenesis of alcohol-induced tissue damage and thus a focus of considerable research interest (Arteel 2003; Bondy 1992; Cederbaum 2001; Wu and Cederbaum 2003). Ingestion of alcohol causes oxidative injury (Reinke et al. 1987; Salem et al. 2006) and accumulation of some toxic, non-oxidative ethanol metabolites in various organs such as heart, liver, and muscle (Salem et al. 2006). One of the primary cellular defense mechanisms against alcohol insult is the rapid synthesis of HSP70 (inducible HSP70 or HSP72) (Saika et al. 2000; Tsukimi and Okabe 2001). Previous studies report inconsistent findings on the role of redox imbalance in alcohol HSP72 induction. It has been shown that high doses of ethanol for 1 week (5 g/kg body weight per day) (Calabrese et al. 2000) or 12 weeks (2 g/kg body weight per day) (Calabrese et al. 1998) induce HSP70 protein in rat brain and liver. In contrast, single intraperitoneal injection of 3.5 g/kg alcohol seems ineffective on HSP70 protein content in rat skeletal muscles (Nakahara et al. 2006), although it causes oxidative stress (Gonenc et al. 2005; Scolaro et al. 2012). Thus, alcohol induction of HSP70 may not be explained solely by oxidative stress and may be initiated by additional factors such as cellular structural damage (Porras et al. 2006). To date, few studies have examined alcohol-induced HSP70 changes in various tissues in association with both oxidative status and structural alterations.
Caffeine, a major constituent of coffee, is frequently consumed concurrently with or immediately after alcohol. Caffeinated alcoholic drinks have become increasingly available (Marczinski and Fillmore 2006), and alcoholic beverages mixed with energy drinks containing caffeine are also common in restaurant bars. There is wide concern about the potential health risks associated with consumption of caffeinated alcoholic beverages (Attwood 2012; Weldy 2010). Research findings remain inconsistent about the interactive effects of caffeine and alcohol as caffeine has been reported to antagonize or potentiate actions of alcohol (Fudin and Nicastro 1988). Furthermore, it remains undetermined how caffeine affects alcohol-induced cell injury, oxidative stress, and HSP72 synthesis in vivo although caffeine may increase plasma HSP72 (Fortes and Whitham 2011; Whitham et al. 2006).
In the present study, we hypothesized that short-term administration of high-dose alcohol would induce tissue injury, oxidative stress, and synthesis of tissue HSP72, and the concurrent use of high-dose caffeine would not affect alcohol-induced injury. Specifically, we investigated the effects of repeated oral administration (once a day for 10 days) of high-dose alcohol and caffeine, alone and in combination, on heart, liver, and gastrocnemius muscle of mice. Inflammatory and oxidative activities and HSP72 levels along with structural changes were assessed in these tissues. Previous studies have shown that the 10-day alcohol regimen is effective for inducing oxidative stress and upregulation of stress proteins (Bae et al. 2011; Kessova and Cederbaum 2007; Ki et al. 2010; Qin and Crews 2012) and causing chronic-binge effects in mice (Ki et al. 2010).
Materials and methods
Animals
Male C57BL/6J mice (6 weeks old) were obtained from Jackson Laboratories, Bar Harbor, ME and were housed at the Uniformed Services University of the Health Sciences (USUHS) animal facility. All procedures were approved by the USUHS Institutional Animal Care and Use Committee. Animals were allowed ad libitum food and water on a standard chow. At least 1 week acclimatization period was given, and all animals were healthy as evidenced by weight gain and normal behavioral activity before proceeding with experimental treatments (n = 6).
Chemicals
All chemicals, phosphate-buffered saline (PBS, pH 7.4), caffeine, and ethanol were purchased from Sigma-Aldrich Corporation (St. Louis, MO). Cytokine Mouse 20 Plex Luminex kits were purchased from Invitrogen (Carlsbad, CA), and 8-isoprostane enzyme-linked immunosorbant assay (ELISA) and 8-isoprostane hydrolization kits were purchased from Cayman Chemicals (Ann Arbor, MI).
Experimental groups
Animals were divided into four different treatment groups: vehicle (distilled water), alcohol (OH), caffeine (Caf), and alcohol plus caffeine (OH + Caf). Mice were gavaged with 0.2 ml of treatment for ten consecutive days in the morning (between 0700 and 0800 hours) by using a 22-gauge mouse feeding needle (1 in. curved). The feeding content contained water (vehicle), 25 % ethanol (OH), 1.2 mg caffeine (Caf), or 25 % ethanol plus 1.2 mg caffeine (OH + Caf). The daily doses were approximately 1.7 g/kg alcohol and 44 mg/kg caffeine. Mice were food-deprived over night following the 10 days of treatment and anesthetized for tissue collection the morning after.
Sample collection
Mice were anesthetized using Inactin (Sigma-Aldrich, Inc. St. Louis, MO) 100 mg/kg, i.p., and blood was withdrawn from a carotid artery via a catheter. Blood was collected in heparinized 1.5-ml Eppendorf tubes, immediately centrifuged, and isolated plasma was snap frozen in liquid nitrogen. Following blood collection, urine, heart (left ventricle), liver, and gastrocnemius muscles were harvested, washed/cleaned in ice-cold PBS, dissected for protein or histological testing, and snap frozen in liquid nitrogen. All samples were stored at −80 °C until ready for experimental use.
Histology
Following treatment protocol, mice tissues were harvested under general anesthesia for histological examination. The tissues were fixed in 4 % para-formaldehyde after harvesting and dehydrated in increasing concentrations of ethanol, cleared with xylene, and embedded in paraffin blocks. Longitudinal tissue sections were cut 30 μm in thickness, deparafinized, and rehydrated in decreased concentrations of ethanol. The sections were then stained with hematoxylin and eosin, and dehydrated in ethanol. The Leica ST4020 (Leica Microsystems Inc., Buffalo Grove, IL, USA) automated slide stainer was used according to manufacturer’s recommendations: slides were cleared using xylene and mounted with coverslip. Slides were viewed under a microscope at X40 magnification and representative photomicrographs taken.
Plasma analysis
Plasma samples were hydrolyzed before measurement of total free 8-isoprostane by using an 8-isoprostane hydrolyzation batch assay kits as per the manufacturer’s instructions. Hydrolyzed samples were then resuspended in PBS and immediately analyzed. Plasma superoxide dismutase (SOD) capacity was determined based on inhibition of superoxide anion produced by xanthine oxidase (Hapner et al. 2010). Lucigenin (5 μM)-enhanced chemiluminescence was used to assess changes in xanthine oxidase-dependent superoxide. The reaction was initiated by adding xanthine (100 μM) to PBS solutions containing xanthine oxidase and plasma samples in a tube luminometer (Berthold AutoLumat Plus LB 953) at room temperature. The chemiluminescence signal was adjusted to background and continuously measured at 2 Hz. The measurements were performed in duplicate. Inhibition of superoxide was calculated as relative reductions in chemiluminescence, and plasma SOD activity was calculated from a standard curve of inhibition of superoxide by Cu/Zn SOD (Sigma-Aldrich, St. Louis, MO). All plasma samples were run in the same assays to prevent inter-assay variability.
Tissue processing and analysis
Frozen samples were placed into polypropylene test tubes containing 1 ml of ice-cold PBS and homogenized (5–10 s) using a Tissue Tearor (Bartlesville, OK) tissue homogenizer. Homogenates were transferred to preweighed 1.5-ml Eppendorf tubes and centrifuged at 14,000 rpm for 3 min. The supernatants were placed into new 1.5-ml Eppendorf tubes and frozen at −80 °C prior to use in assays. The remaining pellet was evaporated to dryness and weighed for sample data analysis correction.
Mouse cytokine 20-plex panel kit (Invitrogen, Camarillo, CA) was used to assess fibroblast growth factor (FGF) basic, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, interleukin (IL)-1α, IL-1β, IL-2,IL-4, IL-5, IL-6, IL-10, IL-12p40p70, IL-13, IL-17, IP-10, KC, monocyte chemotactic protein-1 (MCP-1), monokine induced by interferon-gamma (MIG), macrophage inflammatory protein-1α (MIP-1α), tumor necrosis factor-alpha (TNF-α), and vascular endothelial growth factor (VEGF). The assay was performed with Luminex 100 (Luminex Corporation, Austin, TX) according to manufacturer’s instructions. Data analysis was performed using the Master-Plex QT software.
Quantitative RT-PCR
RNA was isolated from tissue using RNeasy Mini Kit and QIAshredder (Qiagen, Valencia, CA), and cDNA templates were prepared with the Moloney murine leukemia virus reverse transcriptase directed iScript One-Step reverse transcription polymerase chain reaction (RT-PCR) system (Hercules, CA). The following primers were synthesized by the Genomics Core at the Biomedical Instrumentation Center (USUHS, MD) and utilized for real-time RT-PCR: HSP72 (5-CTCCCTCTTGCGTTGCCTC-3 and 5-ACCCGCAGTAATAGCCATCTG-3), and glyceraldehyde-3-phosphate dehydrogenase (5-TGACAACTTTGGTATCGTGGAAGG-3 and 5-AGGGATGATGTTCTGGAGAGCC-3). Real-time RT-PCR was performed for 40 cycles using the Bio-Rad iCycler iQ real time PCR thermocycler and iScript SYBR green PCR supermix (Hercules,CA). Quantification of the RT-PCR products normalized to glyceraldehyde-3-phosphate dehydrogenase expression was performed using iCycler iQ data analysis software.
Western blot analysis
Homogenized tissue samples (25 μl, equivalent to 50 μg protein) were subjected to denaturing and reducing gel electrophoresis on BioRad 4–15 % Tris–HCl (10 well/50 μl) precast Mini-Protean TGX gel cassettes using a BioRad Mini-Protean Tetra Cell module at 200 V for 45 min in BioRad Tris glycine/SDS buffer (25 mM Tris, 192 mM glycine, and 0.1 % SDS), followed by electrophoretic blotting onto BioRad nitrocellulose membrane (0.2 μm) using a BioRad Trans-Blot Turbo transfer system (Hercules, CA). HSP72 protein was detected using a primary mouse anti-HSP72 antibody (Santa Cruz, CA) diluted 1:200 and a horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody (GE Life Science, NJ) diluted 1:1,000. Selected blots were reprobed using a β-actin monoclonal antibody diluted 1:200 (Santa Cruz, CA) to assess gel well loading efficiency.
ELISA
Tissue homogenate supernatants were measured in duplicates using commercial ELISA kits sensitive to murine samples. The following ELISA kits were used for HSP72 (Stressgen, Ann Arbor, MI) and HSF1 (Enzo Life Sciences, Plymouth, PA) as per manufacturers’ instructions. The aspirating and washing cycles were completed by using an automatic microplate washer (Tecan Group Ltd, Switzerland). Samples were analyzed using the Magellan Data Analysis System (Tecan, Austria) and normalized to dry tissue weight (dw). Sensitivities of the assays were 35 and 60 pg/ml, respectively. Intra- and inter-assay coefficients of variation for ELISA concentrations were less than 5 % per assay.
Statistical analysis
Data are expressed as mean ± SEM. One-way ANOVA with Bonferroni’s post hoc test was used for comparison. The results were considered significant at P ≤ 0.05.
Results
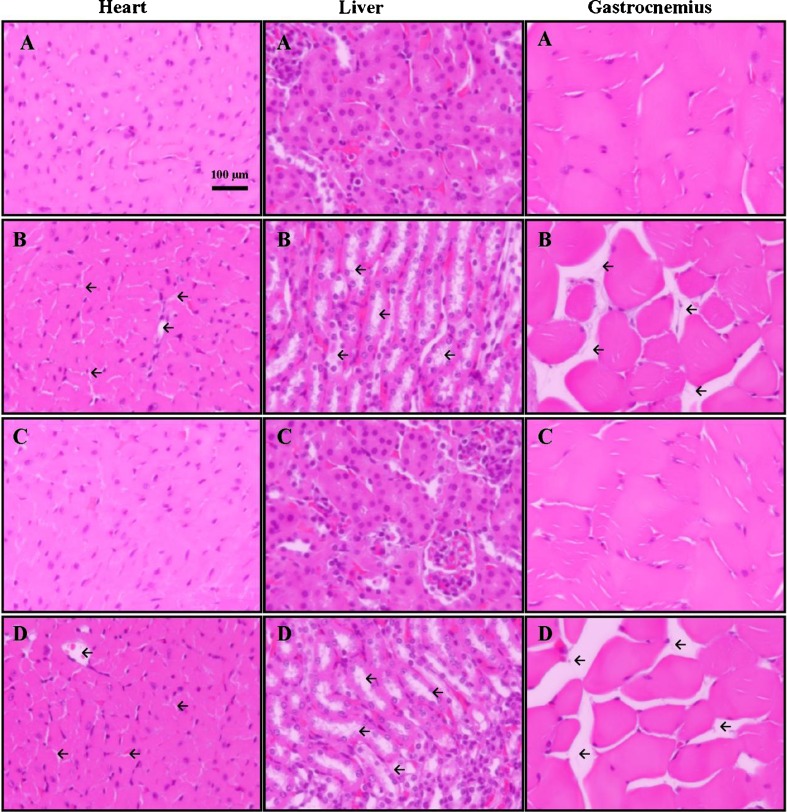
Administration of alcohol resulted in significant morphological changes in heart, liver, and gastrocnemius muscles (Fig. 1). Mice treated with alcohol and a combination of alcohol and caffeine exhibited areas of mild to moderate structural lesions/degeneration in heart, liver, and gastrocnemius muscle tissue sections. Mice treated with caffeine or vehicle only did not exhibit any such specific histological abnormalities. Furthermore, no significant differences in food/water intake or body weight were observed between the different mice groups over the 10 days treatment period (data not shown).
Fig. 1.
Representative photomicrographs of gastrocnemius muscle, heart, and liver tissue from C57BL/6J mice treated with vehicle (A), alcohol (B), caffeine (C), and alcohol + caffeine (D). Longitudinal tissue sections (30 μm) were stained with hematoxylin and eosin for microscopic evaluation at ×40 magnification. Areas of tissue lesion/degeneration are denoted by the inserted arrows
Administration of alcohol caused a significant reduction in plasma SOD concentration (P < 0.05, power = 0.97, n = 6) compared to vehicle (Table 1); in contrast, caffeine administration resulted in significant increases in plasma 6-isoprostane concentration (P < 0.05, power = 0.89, n = 6) compared to vehicle. No other changes in cytokines were noted. Moreover, the following cytokines were undetectable or inconsistently detectable in plasma samples and thus not reported: GM-CSF, IFN-γ, IL-1α, IL-2, IL-4, IL-5, IL-13, IL-17, IP-10, KC, MCP-1, MIG, MIP-1α, and TNF-α.
Table 1.
Comparison of plasma profiles in mice treated with vehicle, alcohol (OH), caffeine (Caf), and combined alcohol and caffeine (OH + Caf)
| Vehicle | OH | Caf | OH + Caf | |
|---|---|---|---|---|
| SOD (units/ml) | 29.1 ± 1.9 | 22.6 ± 1.8* | 29.1 ± 0.1 | 26.3 ± 1.1 |
| 8-Isoprostane (pg/ml) | 52.5 ± 12.0 | 51.4 ± 22.3 | 109.0 ± 23.7* | 41.3 ± 7.2 |
| Cytokines (pg/ml) | ||||
| FGF basic | 81.4 ± 9.8 | 84.6 ± 15.9 | 112 ± 19 | 95.8 ± 9.7 |
| IL-1β | 10.2 ± 4.6 | 10.4 ± 4.8 | 13.0 ± 7.3 | 14.1 ± 4.1 |
| IL-6 | 1.6 ± 0.5 | 0.9 ± 0.3 | 1.4 ± 0.9 | 0.4 ± 0.2 |
| IL-10 | 152 ± 29 | 156 ± 29 | 189 ± 1 | 188 ± 20 |
| IL-12 | 32.7 ± 10.3 | 14.5 ± 5.5 | 23.4 ± 2.2 | 27.0 ± 4.9 |
| VEGF | 21.6 ± 7.5 | 10.6 ± 3.1 | 19.4 ± 13.2 | 27.1 ± 7.1 |
*P < 0.05, compared to vehicle, n = 6 per group
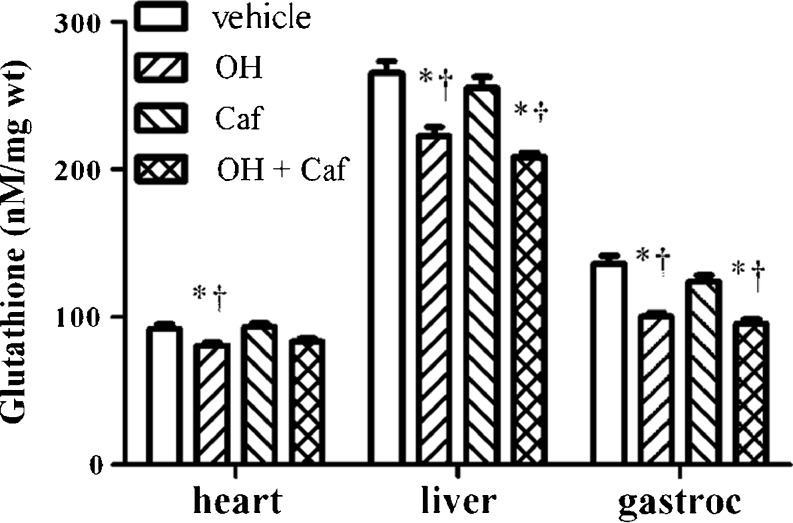
As shown in Fig. 2, overall there were significant group effects on glutathione in heart (P = 0.005), liver (P < 0.0001), and gastrocnemius muscle (P < 0.0001). Post hoc analysis revealed that administration of alcohol resulted in significantly lower glutathione concentrations in mouse heart, liver, and gastrocnemius muscle compared to that treated with vehicle (P < 0.001, power > 0.8) and caffeine (P < 0.001, power > 0.8). Similar reductions were found in liver and gastrocnemius muscles, but not in heart, when alcohol was administered with caffeine (p < 0.01). In contrast, administration of caffeine alone had no significant effects on glutathione concentrations in these tissues.
Fig. 2.
Comparison of total glutathione concentrations in heart, liver, and gastrocnemius muscle (gastroc) of mice (n = 6 per group) treated with vehicle, alcohol (OH), caffeine (Caf), and combined alcohol and caffeine (OH + Caf). *P < 0.05, compared to vehicle; †P < 0.05, compared to Caf
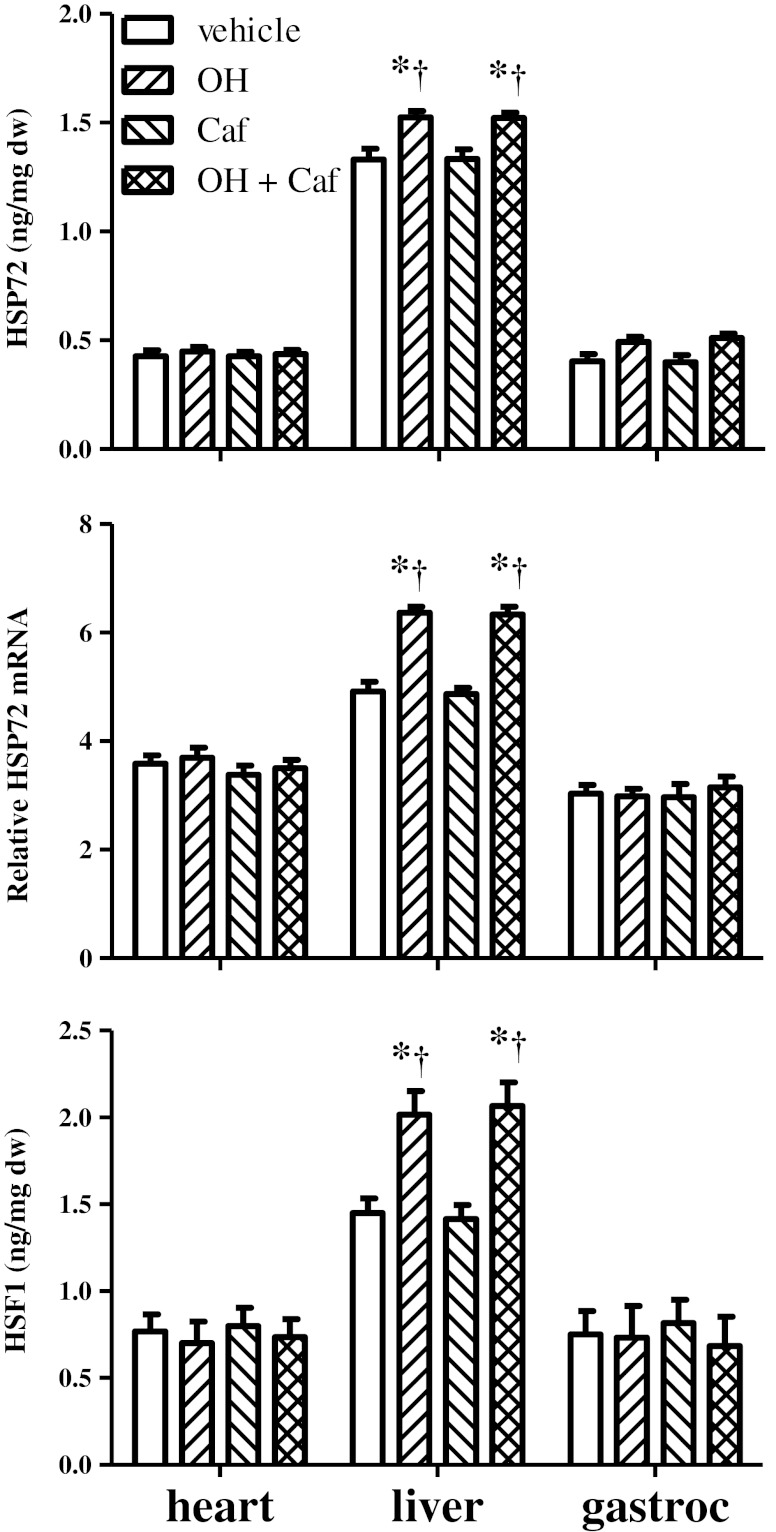
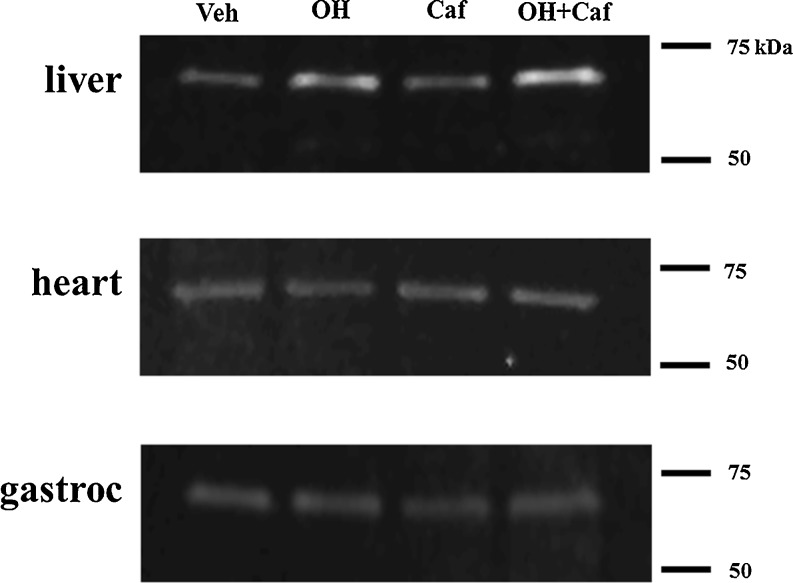
A significant group effect on HSP72 content was noted for liver (P = 0.0006), but not for heart and gastrocnemius muscle (Fig. 3). Post hoc analysis of ELISA data revealed that mice treated with alcohol or alcohol plus caffeine showed significant increases in HSP72 protein in liver compared to mice treated with vehicle or caffeine alone (P < 0.001, power > 0.8). A similar pattern was also observed for HSP72 mRNA levels (P < 0.001, power > 0.8) and HSF1 protein (P < 0.01, power > 0.8). However, mice treated with caffeine alone exhibited no significant alterations in HSP72 or HSF1 levels in these tissues. The changes in HSP72 were further confirmed using Western blot analysis (Fig. 4).
Fig. 3.
Comparison of HSP72 protein and mRNA and HSF1 levels in heart, liver, and gastrocnemius muscle (gastroc) of mice (n = 6 per group) treated with vehicle, alcohol (OH), caffeine (Caf), and combined alcohol and caffeine (OH + Caf). *P < 0.05, compared to vehicle; †P < 0.05, compared to Caf
Fig. 4.
Representative Western blot showing the expression of HSP72 in heart, liver, and gastrocnemius (gastroc) muscle of mice treated with vehicle (Veh), alcohol (OH), caffeine (Caf), and combined alcohol and caffeine (OH + Caf)
Discussion
It is well established that excessive alcohol consumption can result in damage to multiple tissues, including liver, heart, and skeletal muscle (Duguay et al. 1982; Preedy et al. 1999, 2007). In the present study, we demonstrated that short-term administration of high-dose alcohol alone induced oxidative stress, as evidenced by the lesions and reductions of glutathione in the tested tissues. The stress response induced by alcohol seemed to be much greater in liver compared to cardiac and skeletal muscles as demonstrated by upregulation of HSP72 in liver but not in other tissues. Importantly, the effects of alcohol were not exacerbated by concurrent administration of high-dose caffeine. Short-term administration of high-dose caffeine alone did not produce oxidative alterations or induce stress responses in any of the tissues tested. In fact, caffeine appeared to provide slight protection against antioxidant depletion (i.e., reductions of SOD in plasma and glutathione in heart) caused by alcohol alone.
Chronic alcohol consumption is often associated with tissue degeneration changes, such as cirrhosis (Diehl et al. 1990; Duguay et al. 1982; Wands et al. 1979, 1980) and myopathy (Iacovoni et al. 2010; Preedy et al. 2007). Ethanol is rapidly absorbed (20 % in the stomach and 80 % in the small intestine) upon consumption and results in uniform distribution within body tissues (Gemma et al. 2006). Ethanol is mainly metabolized by alcohol dehydrogenase in the liver, with acetaldehyde as the resultant toxic intermediate; this compound is rapidly converted to acetate by nearly every tissue in the body. Liver, heart, and muscles are among the organs/tissues frequently affected by alcohol (Preedy et al. 1999). Alcohol has been shown to cause cellular injury by impairing protein synthesis in various tissues (Lang et al. 2001; Preedy et al. 2003). This study confirms that short-term administration of alcohol causes cellular lesions or damage in heart, liver, and skeletal muscle.
Oxidative stress has been implicated in the pathogenesis of alcohol-induced tissue injury (Cederbaum et al. 2009; Das and Vasudevan 2007; Preedy et al. 1996). Our results demonstrate that ten consecutive days of alcohol administration induced significant oxidative stress in mice, as evidenced by cellular damage and reduced glutathione levels in all tested tissues, as well as decreased plasma SOD. Glutathione is among the most abundant, soluble, antioxidant molecules in mammalian cells and serves an important role in the anti-oxidation of reactive oxygen species and free radicals (Bray and Taylor 1993; Mytilineou et al. 2002). Numerous studies have demonstrated that decreased levels of glutathione are associated with cellular damage (Dam et al. 2012; Yamada et al. 2011) and alcohol reduces glutathione concentrations (Otis and Guidot 2010; Waly et al. 2011) in various tissues.
In contrast, studies remain inconsistent on how caffeine, a natural component of coffee, mediates oxidative stress. Caffeine indeed can exhibit both antioxidant and pro-oxidant properties (Azam et al. 2003). For example, caffeine administration has been shown to both reduce (Farag and Abdel-Meguid 1994) and increase tissue glutathione levels (Aoyama et al. 2011), as well as minimize the depletion of glutathione induced by oxidative stress (Varma et al. 2010). These contradictory effects on tissue oxidation are likely attributable to different caffeine concentrations. This dose-dependent protective effect of caffeine could be explained by recent mouse studies wherein high caffeine doses (50–100 mg/kg) inhibited pro-inflammatory cytokine processes and protected against major organ and tissue damage, while lower caffeine doses (<20 mg/kg) exacerbated organ damage by promoting pro-inflammatory cytokine processes (Li et al. 2011; Ohta et al. 2007). In this study, we demonstrated that short-term caffeine intake at the high dose of ∼44 mg/kg did not promote oxidative injury and may have improved the availability of antioxidants depleted by alcohol intake.
Induction of stress proteins is one of the primary cellular defense mechanisms against alcoholic injury (Saika et al. 2000; Tsukimi and Okabe 2001). Tissue injury (Porras et al. 2006) and oxidative stress (Calabrese et al. 2000) have been suggested to contribute to the induction of cellular HSP70s by alcohol. Glutathione depletion has also been shown to serve as an oxidative stress signal to induce HSP70 (Callahan et al. 2002; Liu et al. 1996; McDuffee et al. 1997). Our findings that HSP72 was significantly increased only in liver, and not in heart and gastrocnemius muscle, could not be simply explained by the above factors because oxidative injury and glutathione depletion occurred in all the tested tissues. Unlike other organs, the liver is the site where approximately 95 % of ingested alcohol undergoes metabolic degradation (Baillie 1971) and acts as the “first line” in the defense against the effects of alcohol following its oral administration. Thus, induction of HSP72 in liver by alcohol could likely be a result of direct exposure to alcohol metabolites, although this would require further testing for confirmation. Alternatively, our results may suggest that liver is more sensitive to induction of HSP72 by alcohol than other tissues.
It is interesting that the heart was less sensitive than liver and skeletal muscle to the insult and oxidation induced by alcohol—alone or in combination with caffeine. Several animal studies have shown that administration of alcohol may indeed impart selective cardiac protection (Abou-Agag et al. 2005; Krenz et al. 2001; Zhou et al. 2002). This may explain, to some extent, why both the glutathione depletion and HSP72 induction in our study were less severe for the heart compared to liver and gastrocnemius muscle. This observation would require functional studies to be supported fully.
As this study used a mouse model, our findings are not directly relevant to human populations at large. In addition, the dose effects of both alcohol and caffeine in mice would be expected to vary somewhat to that produced in humans. Our use of 44 mg/kg of caffeine is comparable to other doses reported in the literature, but far higher than what humans would be expected to consume. Likewise, the dose of alcohol used—1.7 g/kg—is common among studies using “high-metabolism” species, such as rodents, and therefore would most likely not represent alcohol levels achieved in humans within the settings of social drinking. The dose used would equate to a 70 kg person having six pints of beer or two bottles of wine at one sitting, far more than would be typical. Nonetheless, the nature of the observed biochemical and histological changes, in particular the protective effects of high dose caffeine used in combination with alcohol, provide valuable insight into future human clinical studies assessing the effects of caffeine and alcohol intake.
In conclusion, the findings of the present study support the notion that short-term administration of alcohol induces oxidative stress and tissue injury in liver, heart, and gastrocnemius muscle. It is unclear whether induction of HSP72 was mediated through direct exposure to alcohol metabolites as its levels only increase in liver and not in other organs. Clearly, activation of HSP72 in liver by alcohol involved both transcriptional and translational pathways. Concurrent use of high-dose caffeine did not amplify alcohol-induced oxidative damage but instead prevented the depletion of antioxidants as noted by alcohol administration alone. Further studies are needed to confirm the potential protective effect of high-level caffeine consumption against alcohol insult.
Acknowledgments
We consulted Dr. Cara Olsen, Department of Preventive Medicine and Biometrics, Uniformed Services University of the Health Sciences (USUHS), for statistical analysis. This work was supported by USUHS Grant R091EH and the Office of Naval Research Grant N0001411MP20025.
References
- Abou-Agag LH, Khoo NK, Binsack R, et al. Evidence of cardiovascular protection by moderate alcohol: role of nitric oxide. Free Radic Biol Med. 2005;39:540–548. doi: 10.1016/j.freeradbiomed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Matsumura N, Watabe M, et al. Caffeine and uric acid mediate glutathione synthesis for neuroprotection. Neuroscience. 2011;181:206–215. doi: 10.1016/j.neuroscience.2011.02.047. [DOI] [PubMed] [Google Scholar]
- Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–790. doi: 10.1053/gast.2003.50087. [DOI] [PubMed] [Google Scholar]
- Attwood AS. Caffeinated alcohol beverages: a public health concern. Alcohol Alcohol. 2012;47:370–371. doi: 10.1093/alcalc/ags062. [DOI] [PubMed] [Google Scholar]
- Azam S, Hadi N, Khan NU, Hadi SM. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med Sci Monit. 2003;9:BR325–BR330. [PubMed] [Google Scholar]
- Bae SH, Sung SH, Cho EJ, et al. Concerted action of sulfiredoxin and peroxiredoxin I protects against alcohol-induced oxidative injury in mouse liver. Hepatology. 2011;53:945–953. doi: 10.1002/hep.24104. [DOI] [PubMed] [Google Scholar]
- Baillie M. Alcohol and the liver. Gut. 1971;12:222–229. doi: 10.1136/gut.12.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy SC. Ethanol toxicity and oxidative stress. Toxicol Lett. 1992;63:231–241. doi: 10.1016/0378-4274(92)90086-Y. [DOI] [PubMed] [Google Scholar]
- Bray TM, Taylor CG. Tissue glutathione, nutrition, and oxidative stress. Can J Physiol Pharmacol. 1993;71:746–751. doi: 10.1139/y93-111. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Renis M, Calderone A, et al. Stress proteins and SH-groups in oxidant-induced cellular injury after chronic ethanol administration in rat. Free Radic Biol Med. 1998;24:1159–1167. doi: 10.1016/S0891-5849(97)00441-3. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Testa G, Ravagna A, et al. HSP70 induction in the brain following ethanol administration in the rat: regulation by glutathione redox state. Biochem Biophys Res Commun. 2000;269:397–400. doi: 10.1006/bbrc.2000.2311. [DOI] [PubMed] [Google Scholar]
- Callahan MK, Chaillot D, Jacquin C, et al. Differential acquisition of antigenic peptides by Hsp70 and Hsc70 under oxidative conditions. J Biol Chem. 2002;277:33604–33609. doi: 10.1074/jbc.M202890200. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI. Introduction-serial review: alcohol, oxidative stress and cell injury. Free Radic Biol Med. 2001;31:1524–1526. doi: 10.1016/S0891-5849(01)00741-9. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- Dam AD, Mitchell AS, Rush JW, Quadrilatero J. Elevated skeletal muscle apoptotic signaling following glutathione depletion. Apoptosis. 2012;17:48–60. doi: 10.1007/s10495-011-0654-5. [DOI] [PubMed] [Google Scholar]
- Das SK, Vasudevan DM. Alcohol-induced oxidative stress. Life Sci. 2007;81:177–187. doi: 10.1016/j.lfs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Thorgeirsson SS, Steer CJ. Ethanol inhibits liver regeneration in rats without reducing transcripts of key protooncogenes. Gastroenterology. 1990;99:1105–1112. doi: 10.1016/0016-5085(90)90631-a. [DOI] [PubMed] [Google Scholar]
- Duguay L, Coutu D, Hetu C, Joly JG. Inhibition of liver regeneration by chronic alcohol administration. Gut. 1982;23:8–13. doi: 10.1136/gut.23.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag MM, Abdel-Meguid EM. Hepatic glutathione and lipid peroxidation in rats treated with theophylline. Effect of dose and combination with caffeine and acetaminophen. Biochem Pharmacol. 1994;47:443–446. doi: 10.1016/0006-2952(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Fortes MB, Whitham M. Salivary Hsp72 does not track exercise stress and caffeine-stimulated plasma Hsp72 responses in humans. Cell Stress Chaperones. 2011;16:345–352. doi: 10.1007/s12192-010-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromenty B, Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther. 1995;67:101–154. doi: 10.1016/0163-7258(95)00012-6. [DOI] [PubMed] [Google Scholar]
- Fudin R, Nicastro R. Can caffeine antagonize alcohol-induced performance decrements in humans? Percept Mot Skills. 1988;67:375–391. doi: 10.2466/pms.1988.67.2.375. [DOI] [PubMed] [Google Scholar]
- Gemma S, Vichi S, Testai E. Individual susceptibility and alcohol effects:biochemical and genetic aspects. Ann Ist Super Sanita. 2006;42:8–16. [PubMed] [Google Scholar]
- Gonenc S, Uysal N, Acikgoz O, et al. Effects of melatonin on oxidative stress and spatial memory impairment induced by acute ethanol treatment in rats. Physiol Res. 2005;54:341–348. [PubMed] [Google Scholar]
- Hapner CD, Deuster P, Chen Y. Inhibition of oxidative hemolysis by quercetin, but not other antioxidants. Chem Biol Interact. 2010;186:275–279. doi: 10.1016/j.cbi.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Iacovoni A, De Maria R, Gavazzi A. Alcoholic cardiomyopathy. J Cardiovasc Med (Hagerstown) 2010;11:884–892. doi: 10.2459/JCM.0b013e32833833a3. [DOI] [PubMed] [Google Scholar]
- Kessova IG, Cederbaum AI. Mitochondrial alterations in livers of Sod1−/− mice fed alcohol. Free Radic Biol Med. 2007;42:1470–1480. doi: 10.1016/j.freeradbiomed.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz M, Baines CP, Heusch G, et al. Acute alcohol-induced protection against infarction in rabbit hearts: differences from and similarities to ischemic preconditioning. J Mol Cell Cardiol. 2001;33:2015–2022. doi: 10.1006/jmcc.2001.1465. [DOI] [PubMed] [Google Scholar]
- Lang CH, Kimball SR, Frost RA, Vary TC. Alcohol myopathy: impairment of protein synthesis and translation initiation. Int J Biochem Cell Biol. 2001;33:457–473. doi: 10.1016/S1357-2725(00)00081-9. [DOI] [PubMed] [Google Scholar]
- Li J, Li G, Hu JL, et al. Chronic or high dose acute caffeine treatment protects mice against oleic acid-induced acute lung injury via an adenosine A2A receptor-independent mechanism. Eur J Pharmacol. 2011;654:295–303. doi: 10.1016/j.ejphar.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Biochemical and molecular basis of alcohol-induced injury to liver and other tissues. N Engl J Med. 1988;319:1639–1650. doi: 10.1056/NEJM198812223192505. [DOI] [PubMed] [Google Scholar]
- Liu H, Lightfoot R, Stevens JL. Activation of heat shock factor by alkylating agents is triggered by glutathione depletion and oxidation of protein thiols. J Biol Chem. 1996;271:4805–4812. doi: 10.1074/jbc.271.9.4805. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Clubgoers and their trendy cocktails: implications of mixing caffeine into alcohol on information processing and subjective reports of intoxication. Exp Clin Psychopharmacol. 2006;14:450–458. doi: 10.1037/1064-1297.14.4.450. [DOI] [PubMed] [Google Scholar]
- McDuffee AT, Senisterra G, Huntley S, et al. Proteins containing non-native disulfide bonds generated by oxidative stress can act as signals for the induction of the heat shock response. J Cell Physiol. 1997;171:143–151. doi: 10.1002/(SICI)1097-4652(199705)171:2<143::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord. 2002;8:385–387. doi: 10.1016/S1353-8020(02)00018-4. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Hunter R, Hirano M, et al. Alcohol alters skeletal muscle heat shock protein gene expression in rats: these effects are moderated by sex, raised endogenous acetaldehyde, and starvation. Metabolism. 2006;55:843–851. doi: 10.1016/j.metabol.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Ohta A, Lukashev D, Jackson EK, et al. 1,3,7-trimethylxanthine (caffeine) may exacerbate acute inflammatory liver injury by weakening the physiological immunosuppressive mechanism. J Immunol. 2007;179:7431–7438. doi: 10.4049/jimmunol.179.11.7431. [DOI] [PubMed] [Google Scholar]
- Otis JS, Guidot DM. Procysteine increases alcohol-depleted glutathione stores in rat plantaris following a period of abstinence. Alcohol Alcohol. 2010;45:495–500. doi: 10.1093/alcalc/agq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras N, Strauss M, Rodriguez M, Anselmi G. Hsp70 accumulation and ultrastructural features of lung and liver induced by ethanol treatment with and without L-carnitine protection in rats. Exp Toxicol Pathol. 2006;57:227–237. doi: 10.1016/j.etp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Patel VB, Why HJ, et al. Alcohol and the heart: biochemical alterations. Cardiovasc Res. 1996;31:139–147. [PubMed] [Google Scholar]
- Preedy VR, Reilly ME, Patel VB, et al. Protein metabolism in alcoholism: effects on specific tissues and the whole body. Nutrition. 1999;15:604–608. doi: 10.1016/S0899-9007(99)00096-9. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Ohlendieck K, Adachi J, et al. The importance of alcohol-induced muscle disease. J Muscle Res Cell Motil. 2003;24:55–63. doi: 10.1023/A:1024842817060. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Crabb DW, Farres J, Emery PW. Alcoholic myopathy and acetaldehyde. Novartis Found Symp. 2007;285:158–177. doi: 10.1002/9780470511848.ch12. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation. 2012;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke LA, Lai EK, DuBose CM, McCay PB. Reactive free radical generation in vivo in heart and liver of ethanol-fed rats: correlation with radical formation in vitro. Proc Natl Acad Sci U S A. 1987;84:9223–9227. doi: 10.1073/pnas.84.24.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika M, Ueyama T, Senba E. Expression of immediate early genes, HSP70, and COX-2 mRNAs in rat stomach following ethanol ingestion. Dig Dis Sci. 2000;45:2455–2462. doi: 10.1023/A:1005615714451. [DOI] [PubMed] [Google Scholar]
- Salem RO, Laposata M, Rajendram R, et al. The total body mass of fatty acid ethyl esters in skeletal muscles following ethanol exposure greatly exceeds that found in the liver and the heart. Alcohol Alcohol. 2006;41:598–603. doi: 10.1093/alcalc/agl069. [DOI] [PubMed] [Google Scholar]
- Scolaro B, Delwing-de Lima D, da Cruz JG, Delwing-Dal Magro D. Mate tea prevents oxidative stress in the blood and hippocampus of rats with acute or chronic ethanol administration. Oxid Med Cell Longev. 2012;2012:314758. doi: 10.1155/2012/314758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukimi Y, Okabe S. Recent advances in gastrointestinal pathophysiology: role of heat shock proteins in mucosal defense and ulcer healing. Biol Pharm Bull. 2001;24:1–9. doi: 10.1248/bpb.24.1. [DOI] [PubMed] [Google Scholar]
- Varma SD, Hegde KR, Kovtun S. Oxidative stress in lens in vivo: inhibitory effect of caffeine. A preliminary report. Mol Vis. 2010;16:501–505. [PMC free article] [PubMed] [Google Scholar]
- Waly MI, Kharbanda KK, Deth RC. Ethanol lowers glutathione in rat liver and brain and inhibits methionine synthase in a cobalamin-dependent manner. Alcohol Clin Exp Res. 2011;35:277–283. doi: 10.1111/j.1530-0277.2010.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wands JR, Carter EA, Bucher NL, Isselbacher KJ. Inhibition of hepatic regeneration in rats by acute and chronic ethanol intoxication. Gastroenterology. 1979;77:528–531. [PubMed] [Google Scholar]
- Wands JR, Carter EA, Bucher NL, Isselbacher KJ. Effect of acute and chronic ethanol intoxication on hepatic regeneration. Adv Exp Med Biol. 1980;132:663–670. doi: 10.1007/978-1-4757-1419-7_69. [DOI] [PubMed] [Google Scholar]
- Weldy DL. Risks of alcoholic energy drinks for youth. J Am Board Fam Med. 2010;23:555–558. doi: 10.3122/jabfm.2010.04.090261. [DOI] [PubMed] [Google Scholar]
- Whitham M, Walker GJ, Bishop NC. Effect of caffeine supplementation on the extracellular heat shock protein 72 response to exercise. J Appl Physiol. 2006;101:1222–1227. doi: 10.1152/japplphysiol.00409.2006. [DOI] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277–284. [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Hashida K, Takarada-Iemata M, et al. Alpha-lipoic acid (LA) enantiomers protect SH-SY5Y cells against glutathione depletion. Neurochem Int. 2011;59:1003–1009. doi: 10.1016/j.neuint.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Zhou HZ, Karliner JS, Gray MO. Moderate alcohol consumption induces sustained cardiac protection by activating PKC-epsilon and Akt. Am J Physiol Heart Circ Physiol. 2002;283:H165–H174. doi: 10.1152/ajpheart.00408.2001. [DOI] [PubMed] [Google Scholar]