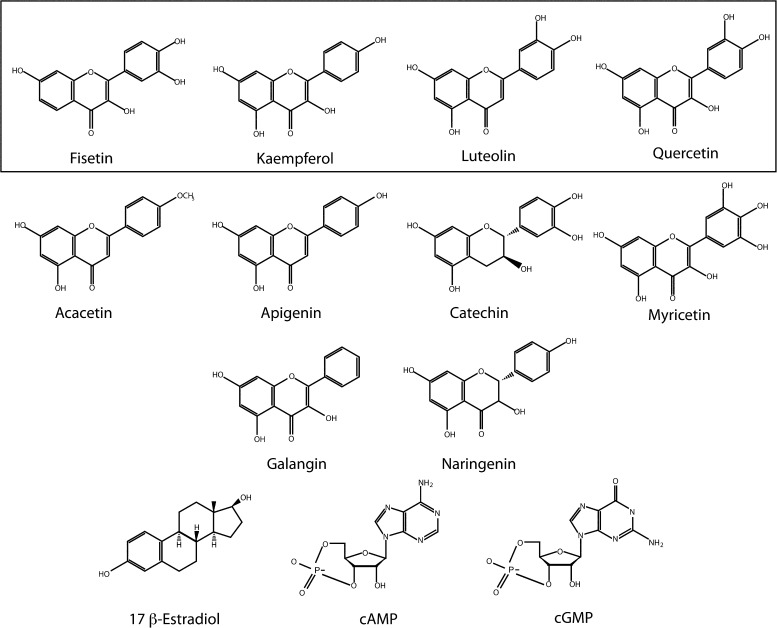
Figure 2.
Chemical structures of screened flavonoids and structurally related compounds. The following flavonoids were included in this screen: fisetin (2-(3,4-dihydroxyphenyl)-3,7-dihydroxychromen-4-one), kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one), luteolin (2-(3,4-dihydroxyphenyl)- 5,7-dihydroxy-4-chromenone), quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one), acacetin (5,7-dihydroxy-2-(4-methoxyphenyl)chromen-4-one), apigenin (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one), catechin ((2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol), myricetin (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4-chromenone), galangin (3,5,7-trihydroxy-2-phenylchromen-4-one), and narnigenin (5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one). The steroid hormone 17-β-estradiol ((17β)-estra-1,3,5(10)-triene-3,17-diol) and the cyclic nucleotides cAMP (3′5′-cyclic adenosine monophosphate) and cGMP (3′5′-cyclic guanosine monophosphate) were also included in this screen. Box highlights the compounds that potentiated mEAG1 currents.