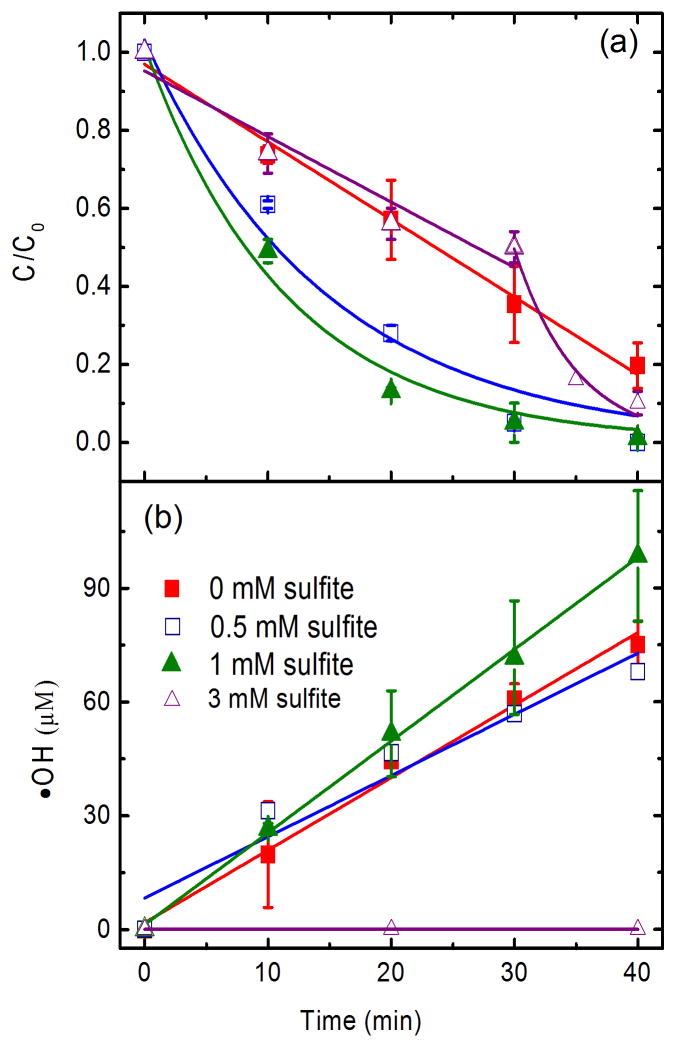
Fig. 4.
Effect of sulfite on (a) TCE degradation and (b) •OH radical accumulation. The degradation conditions for (a) are based on 198 μM initial TCE concentration, pH 4, 13.7 mg/L Fe2+, 1 g/L Pd/Al2O3 and 10 mM Na2SO4 background electrolyte. The reaction conditions for (b) are the same as for (a) except that no TCE was included. Lines and curves in (a) refer to pseudo-zero-order and pseudo-first-order kintic fittings, respectively. Pseudo-zero-order reaction kinetics is given by Ct = C0−k0t, where t is the reaction time (min), k0 is the zero-order rate constant (μM/min), and c0 and ct are the concentrations (μM) at times of t = 0 and t = t, respectively. Pseudo-first-order reaction kinetics is expressed as ln(Ct/C0) = k1t + b, where b is a constant and k1 is the first-order rate constant (min−1).