Fig. 1.
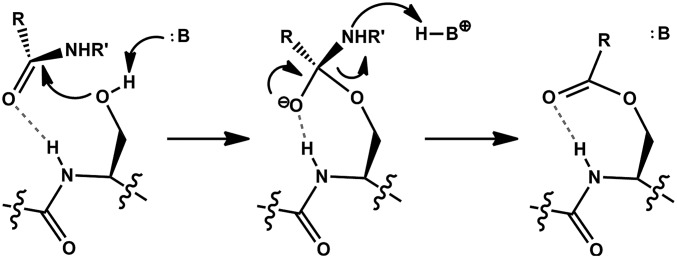
The acylation mechanism of a Ser protease. After substrate binding, the Ser side chain attacks the scissile peptide bond to generate a tetrahedral oxyanion intermediate. Protonation of the amine leaving group allows collapse of the intermediate and formation of an acyl-enzyme species. Dashed bonds represent the hydrogen bond of the oxyanion hole. See text for details.
