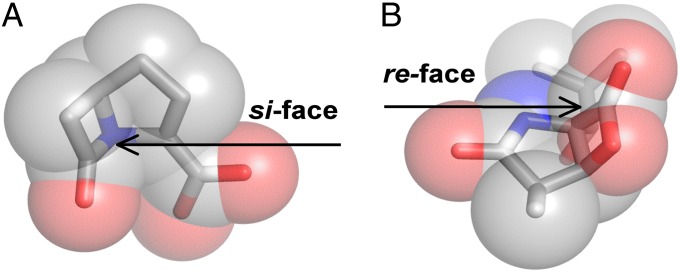
Fig. 8.
Stereospecific antibiotics. (A) The β-lactam molecule carbapenem contains stereochemistry that blocks the si face of the elecrophilic carbonyl and this molecule may be used only to inhibit re-face attacking enzymes. (B) Conversely, omuralide features a β-lactone–γ-lactam core that occludes the re face of the eletrophilic carbonyl. Consequently, this inhibitor scaffold is effective only against si-face–attacking enzymes.