Fig. P1.
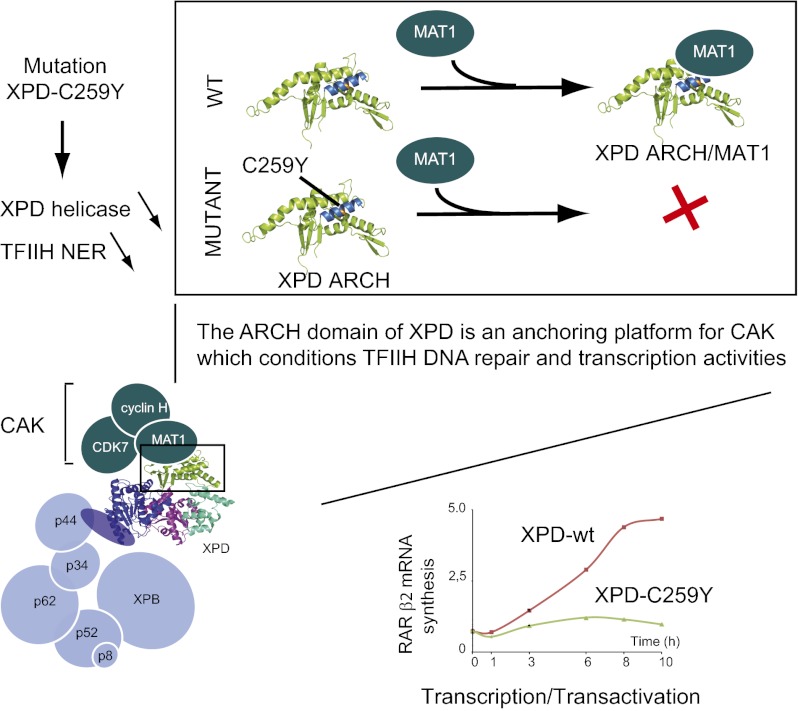
The XPD helicase, a subunit of transcription/DNA repair factor TFIIH, catalyzes the unwinding of damaged DNA during NER and bridges core-TFIIH to the CAK kinase complex. We have established that the ARCH domain of XPD constitutes a platform for the recruitment of CAK through its menage a trois 1 (MAT1) subunit and analyzed the C259Y mutation identified in patients with TTD. This mutation not only affects the interaction with MAT1, thereby decreasing the in vitro transcription/phosphorylation activities of TFIIH itself and impeding the efficient recruitment of the transcription machinery on the promoter of the RARβ2 gene, but also impairs the helicase activity of XPD and the NER activity of TFIIH. Together, these results identify the ARCH domain of XPD as a platform for the recruitment of CAK and show that it plays a key role in DNA recognition and in the regulation of the helicase activity.