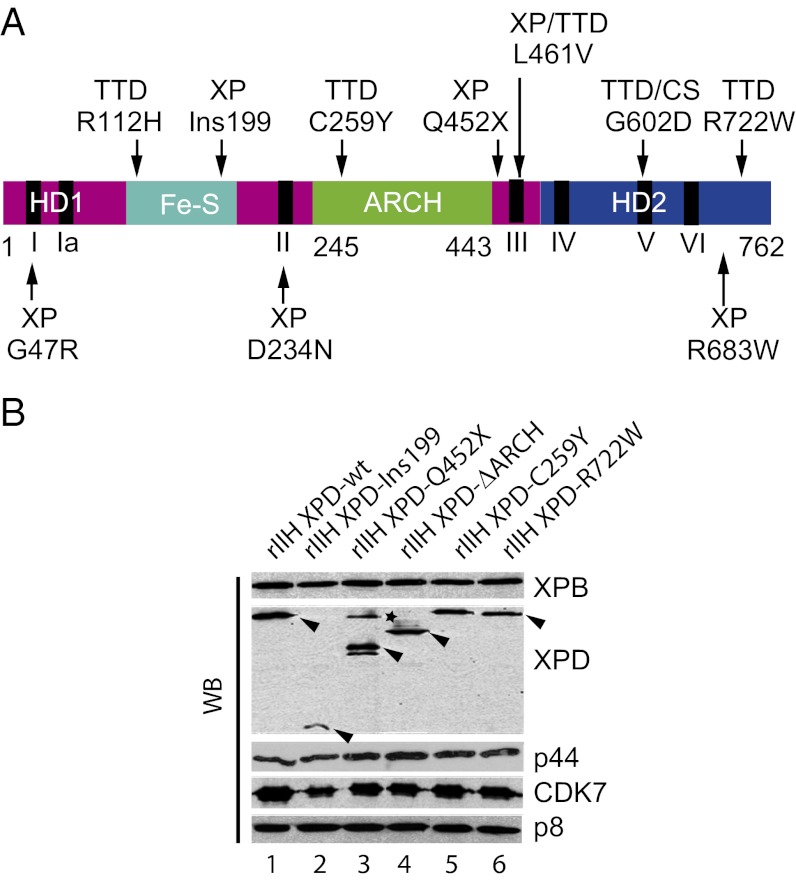
Fig. 1.
TTD and XP mutations in the XPD TFIIH subunit. (A) Schematic representation of XPD. Helicase motor domains HD1 and HD2 are shown in magenta and blue, respectively, the Fe-S iron sulfur-containing domain is in cyan, and the ARCH domain is in green. Black bars indicate the helicase motifs (I, Ia, II, II, IV, V, and VI). Positions of mutations flanking the ARCH domain and diseases associated with the mutations are shown. (B) Production of rIIH. The 10 subunits of human TFIIH, including either wild-type or mutated XPD, were coexpressed in insect cells using the baculovirus expression system, and complexes were immunoprecipitated using an antibody directed against the p44 subunit of the core-TFIIH in low-salt conditions (buffer B containing 75 mM KCl). After elution with a synthetic peptide recognized by Ab-p44, equal amounts of purified rIIHs were analyzed by SDS/PAGE with 12% (wt/vol) polyacrylamide followed by Western blot (WB) analysis with antibodies directed against XPB, the N-terminus of XPD, p44, CDK7, or p8. Arrowheads indicate the theoretical molecular weight of each XPD mutated form. The asterisk indicates a nonspecific band.