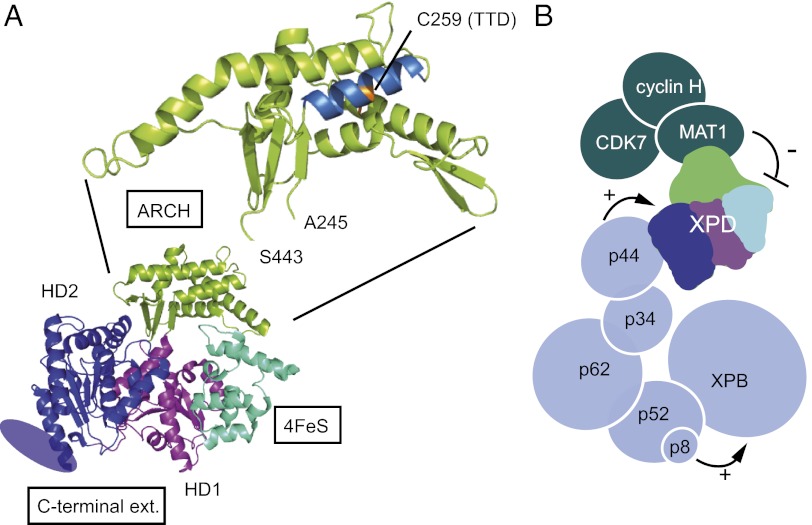
Fig. 6.
XPD structure and TFIIH architecture. (A) Modular organization of XPD. Thermoplasma acidophilum XPD (Protein Data Bank ID code 2VSF) is composed of four structural domains: two RecA-like helicase domains (HD1 in blue and HD2 in magenta), one domain that contains an iron/sulfur group (in cyan), and a domain described as the ARCH domain (in green). The C-terminal extension of eukaryotic XPD that is required for interaction with the p44 core-TFIIH subunit and for which no structural data are available is represented by an ellipse. Residue 259 mutated into a tyrosine in the TTD12PV patient cell line maps onto the first helix of the ARCH domain, and its side chain points into the center of the helical bundle. (B) Schematic of subunit architecture of TFIIH. Each subunit is represented by a circle with the radius of a sphere corresponding to its molecular weight. Interactions between XPB, p52, and p8/TTD-A stimulate XPB ATPase activity and consequently favor binding of TFIIH to damaged DNA. Interactions between p44 and XPD stimulate the helicase activity, allowing unwinding of DNA around the lesion and subsequent double incision by the endonucleases XPF-ERCC1 and XPG. XPD helicase activity, dispensable for transcription initiation but required for NER, is repressed when CAK is associated with TFIIH.