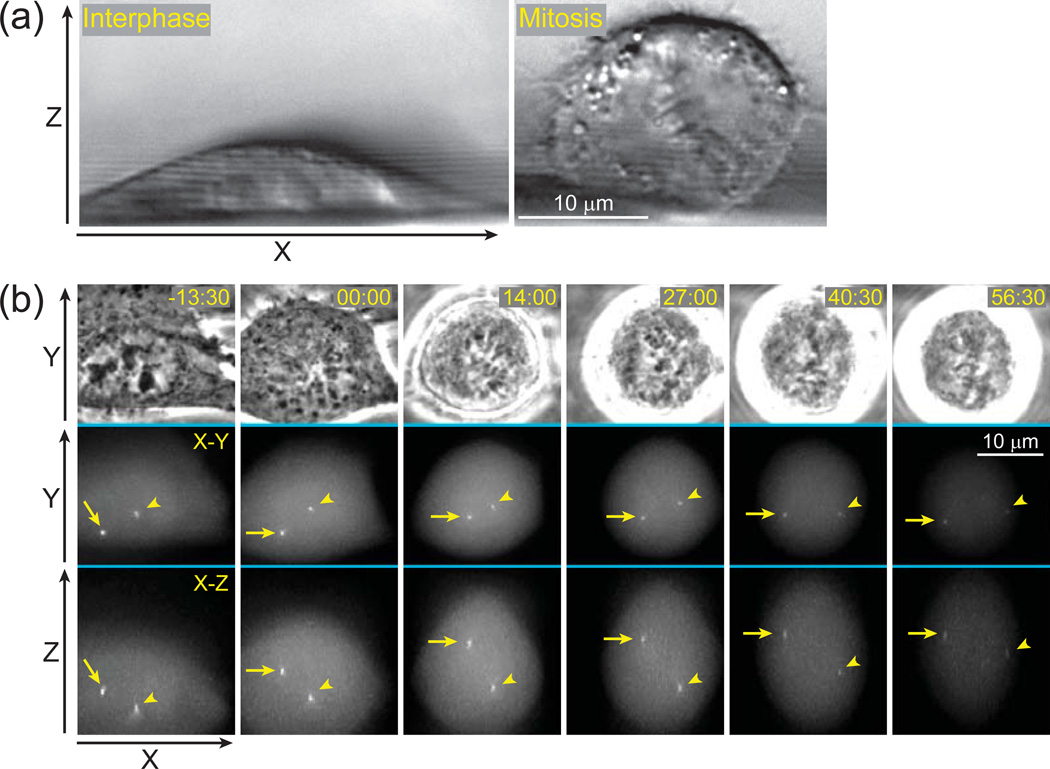
Figure 1.
Conventional approach to 3-D time-lapse imaging results in unnecessary exposure to light. (a) Shape of a typical animal cell changes during the cell cycle. Cultured cells grown on solid substrates are significantly thinner during interphase (left panel) than during mitosis (right panel). Differential Interference Contrast (DIC) images depicting a side-view of human cells (RPE-1) grown on a 120-µm fluorocarbon string mounted on a coverslip. (b) An example of 3-D time-lapse GFP-fluorescence recording (selected frames) in which movements of centrosomes (arrow and arrowhead) are followed in a mitotic human cell (RPE-1). Each time-point is presented as a selected phase-contrast image (top row) and two maximal-intensity projections, one along Z (middle) and one along Y axis (bottom). Although only a few focal planes are required to capture in-focus images of the centrosomes (yellow boxes), a much larger number of focal planes have to be collected (61 planes spanning ~24 µm per time-point) due to gradual rounding of the cell and extensive movements of the centrosomes along Z-axis. These unnecessary focal planes expose cells to additional irradiation resulting in photobleaching (notice gradually decreasing intensity) and phototoxicity (the cell arrests in mitosis). Time in minutes : seconds. Time 00:00 corresponds to nuclear envelope breakdown (onset of mitosis).