Figure 4.
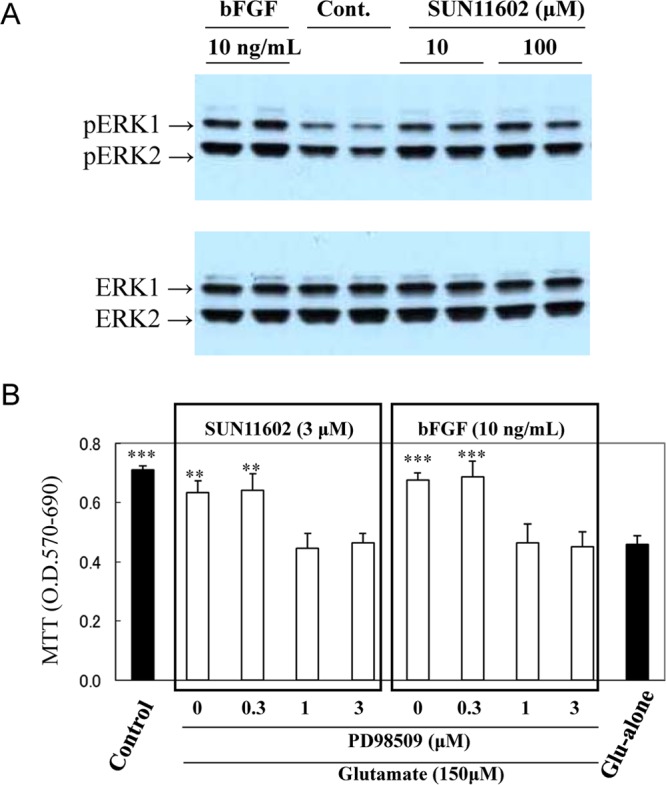
Phosphorylation of extracellular signal-regulated kinase-1/2 (ERK-1/2), which is necessary for the neuroprotection by SUN11602 and bFGF against glutamate-induced toxicity in primary cultures of rat cerebrocortical neurons. (A) Cultured cortical neurons express ERK1/2 proteins at a constant level in normal conditions (lower panel: ERK1, ERK2), whereas, after treatment with 10 or 100 μM SUN11602 and 10 ng/mL bFGF for 20 min, both ERK1/2 proteins of the neurons were phosphorylated substantially (upper panel: p-ERK1, p-ERK2) in comparison to the control lysates. The whole-cell lysates of the cultured neurons were subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunodetected with an antiphosphorylated-ERK1/2 antibody. (B) Inhibition by PD98059 on the neuroprotective effects of SUN11602 and bFGF. Impairment of the ERK kinase/ERK pathway resulted in eliminating the effects of SUN11602 and bFGF, implying that signaling through the pathway plays a pivotal role in activating the neuroprotective mechanisms. The cultures pretreated with PD98059 for 30 min were incubated with SUN11602 or bFGF for 24 h. Thereafter, neurons in the cultures were exposed to 150 μM glutamate for another 24 h, and cell viability was determined by the MTT assay. **p < 0.01, ***p < 0.001 compared to the glutamate group (means ± SEM, n = 6, Dunnett’s multiple comparison test).
