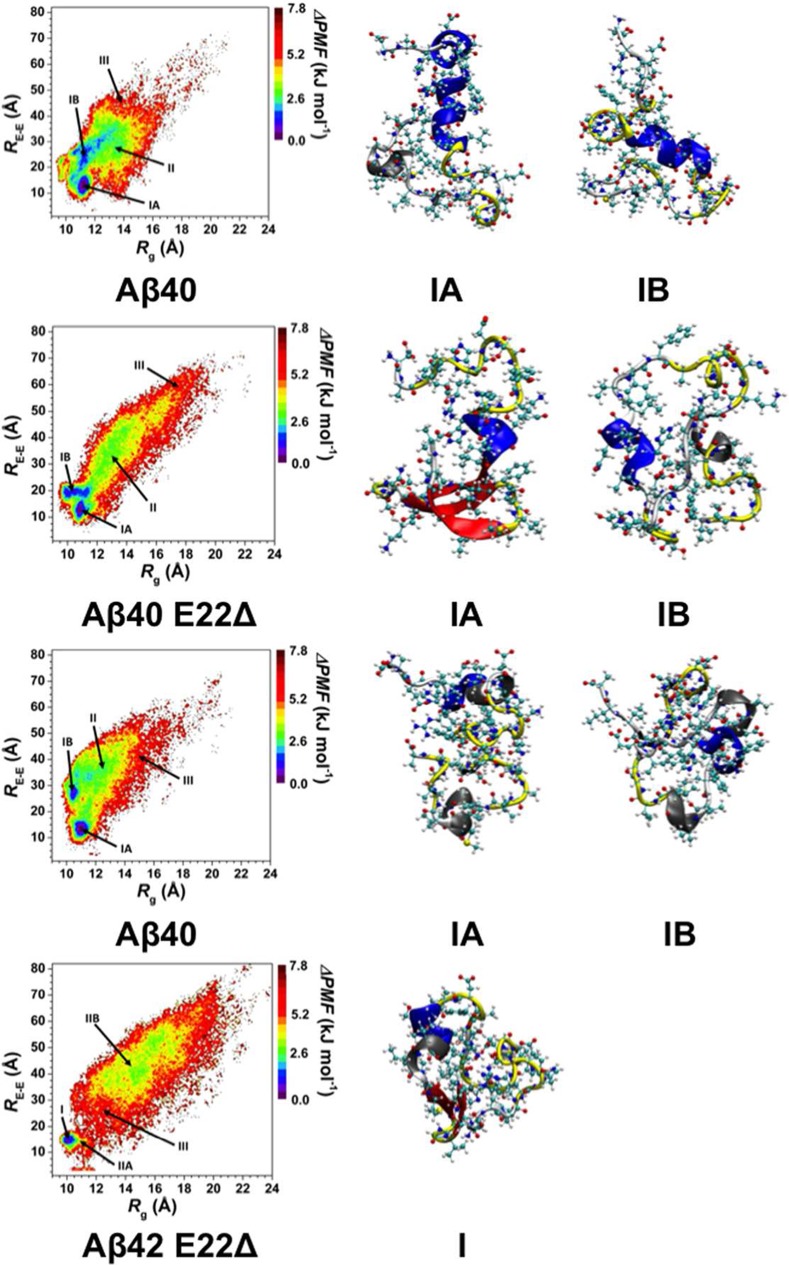
Figure 5.
Change in the potential of mean force (ΔPMF) of the wild-type Aβ40 (Aβ40), E22Δ mutant-type Aβ40 (Aβ40 E22Δ), wild-type Aβ42 (Aβ42), and E22Δ mutant-type Aβ42 (Aβ42 E22Δ) peptides along the coordinates of radius of gyration (Rg) and end-to-end distance (RE-E) in units of kJ mol–1. Structures with representative tertiary and secondary structure characteristics of the most favorable PMF basins (basin IA and IB) for each of the wild- and E22Δ mutant-type Aβ peptides are displayed next to the PMF surface. The secondary structure components of each peptide structure are displayed by the color of the peptide backbone: α-helix (blue), 310-helix (gray), π-helix (purple), β-sheet (red), β-bridge (black), turn (yellow), and coil (white).