Abstract
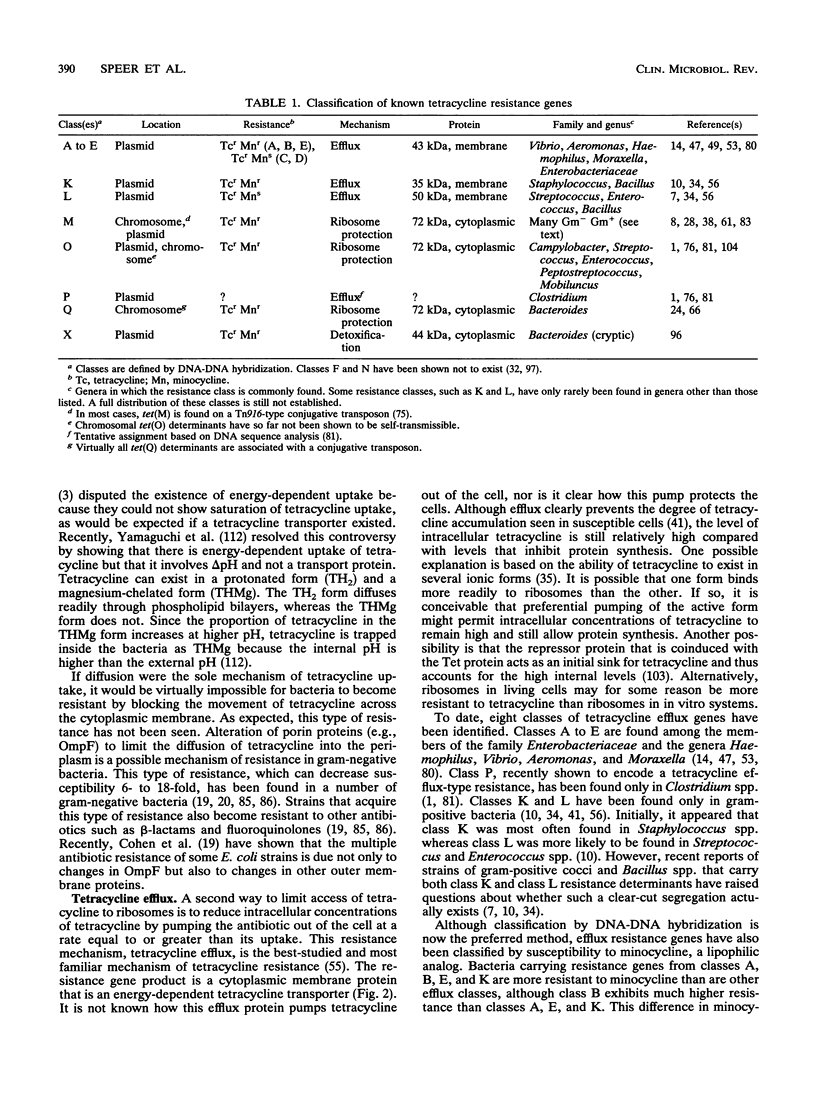
Tetracycline has been a widely used antibiotic because of its low toxicity and broad spectrum of activity. However, its clinical usefulness has been declining because of the appearance of an increasing number of tetracycline-resistant isolates of clinically important bacteria. Two types of resistance mechanisms predominate: tetracycline efflux and ribosomal protection. A third mechanism of resistance, tetracycline modification, has been identified, but its clinical relevance is still unclear. For some tetracycline resistance genes, expression is regulated. In efflux genes found in gram-negative enteric bacteria, regulation is via a repressor that interacts with tetracycline. Gram-positive efflux genes appear to be regulated by an attenuation mechanism. Recently it was reported that at least one of the ribosome protection genes is regulated by attenuation. Tetracycline resistance genes are often found on transmissible elements. Efflux resistance genes are generally found on plasmids, whereas genes involved in ribosome protection have been found on both plasmids and self-transmissible chromosomal elements (conjugative transposons). One class of conjugative transposon, originally found in streptococci, can transfer itself from streptococci to a variety of recipients, including other gram-positive bacteria, gram-negative bacteria, and mycoplasmas. Another class of conjugative transposons has been found in the Bacteroides group. An unusual feature of the Bacteroides elements is that their transfer is enhanced by preexposure to tetracycline. Thus, tetracycline has the double effect of selecting for recipients that acquire a resistance gene and stimulating transfer of the gene.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham L. J., Berryman D. I., Rood J. I. Hybridization analysis of the class P tetracycline resistance determinant from the Clostridium perfringens R-plasmid, pCW3. Plasmid. 1988 Mar;19(2):113–120. doi: 10.1016/0147-619x(88)90050-9. [DOI] [PubMed] [Google Scholar]
- Amano H., Ives C. L., Bott K. F., Shishido K. A limited number of Bacillus subtilis strains carry a tetracycline-resistance determinant at a site close to the origin of replication. Biochim Biophys Acta. 1991 Feb 16;1088(2):251–258. doi: 10.1016/0167-4781(91)90061-p. [DOI] [PubMed] [Google Scholar]
- Argast M., Beck C. F. Tetracycline uptake by susceptible Escherichia coli cells. Arch Microbiol. 1985 Apr;141(3):260–265. doi: 10.1007/BF00408069. [DOI] [PubMed] [Google Scholar]
- Ayoubi P., Kilic A. O., Vijayakumar M. N. Tn5253, the pneumococcal omega (cat tet) BM6001 element, is a composite structure of two conjugative transposons, Tn5251 and Tn5252. J Bacteriol. 1991 Mar;173(5):1617–1622. doi: 10.1128/jb.173.5.1617-1622.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedzyk L. A., Shoemaker N. B., Young K. E., Salyers A. A. Insertion and excision of Bacteroides conjugative chromosomal elements. J Bacteriol. 1992 Jan;174(1):166–172. doi: 10.1128/jb.174.1.166-172.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentorcha F., De Cespédès G., Horaud T. Tetracycline resistance heterogeneity in Enterococcus faecium. Antimicrob Agents Chemother. 1991 May;35(5):808–812. doi: 10.1128/aac.35.5.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram J., Strätz M., Dürre P. Natural transfer of conjugative transposon Tn916 between gram-positive and gram-negative bacteria. J Bacteriol. 1991 Jan;173(2):443–448. doi: 10.1128/jb.173.2.443-448.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K. P., Postle K., Wray L. V., Jr, Reznikoff W. S. Overlapping divergent promoters control expression of Tn10 tetracycline resistance. Gene. 1983 Aug;23(2):149–156. doi: 10.1016/0378-1119(83)90046-x. [DOI] [PubMed] [Google Scholar]
- Bismuth R., Zilhao R., Sakamoto H., Guesdon J. L., Courvalin P. Gene heterogeneity for tetracycline resistance in Staphylococcus spp. Antimicrob Agents Chemother. 1990 Aug;34(8):1611–1614. doi: 10.1128/aac.34.8.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V. Purification and characterization of Tet(M), a protein that renders ribosomes resistant to tetracycline. J Biol Chem. 1991 Feb 15;266(5):2872–2877. [PubMed] [Google Scholar]
- Burdett V. Streptococcal tetracycline resistance mediated at the level of protein synthesis. J Bacteriol. 1986 Feb;165(2):564–569. doi: 10.1128/jb.165.2.564-569.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu-Hoï A., Le Bouguénec C., Horaud T. Genetic basis of antibiotic resistance in Aerococcus viridans. Antimicrob Agents Chemother. 1989 Apr;33(4):529–534. doi: 10.1128/aac.33.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparon M. G., Scott J. R. Excision and insertion of the conjugative transposon Tn916 involves a novel recombination mechanism. Cell. 1989 Dec 22;59(6):1027–1034. doi: 10.1016/0092-8674(89)90759-9. [DOI] [PubMed] [Google Scholar]
- Chopra I., Howe T. G., Linton A. H., Linton K. B., Richmond M. H., Speller D. C. The tetracyclines: prospects at the beginning of the 1980s. J Antimicrob Chemother. 1981 Jul;8(1):5–21. doi: 10.1093/jac/8.1.5. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Flannagan S. E., Ike Y., Jones J. M., Gawron-Burke C. Sequence analysis of termini of conjugative transposon Tn916. J Bacteriol. 1988 Jul;170(7):3046–3052. doi: 10.1128/jb.170.7.3046-3052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Hooper D. C., Wolfson J. S., Levy S. B. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob Agents Chemother. 1989 Aug;33(8):1318–1325. doi: 10.1128/aac.33.8.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Levy S. B. marA locus causes decreased expression of OmpF porin in multiple-antibiotic-resistant (Mar) mutants of Escherichia coli. J Bacteriol. 1988 Dec;170(12):5416–5422. doi: 10.1128/jb.170.12.5416-5422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont H. L., Steele J. H. The human health implication of the use of antimicrobial agents in animal feeds. Vet Q. 1987 Oct;9(4):309–320. doi: 10.1080/01652176.1987.9694119. [DOI] [PubMed] [Google Scholar]
- Epe B., Woolley P., Hornig H. Competition between tetracycline and tRNA at both P and A sites of the ribosome of Escherichia coli. FEBS Lett. 1987 Mar 23;213(2):443–447. doi: 10.1016/0014-5793(87)81539-9. [DOI] [PubMed] [Google Scholar]
- Fletcher H. M., Macrina F. L. Molecular survey of clindamycin and tetracycline resistance determinants in Bacteroides species. Antimicrob Agents Chemother. 1991 Nov;35(11):2415–2418. doi: 10.1128/aac.35.11.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. M., Levy S. B. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a non-plasmid-determined efflux of tetracycline. J Bacteriol. 1983 Aug;155(2):531–540. doi: 10.1128/jb.155.2.531-540.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. A., Hasan T., Hall C. C., Strycharz W. A., Cooperman B. S. Photoincorporation of tetracycline into Escherichia coli ribosomes. Identification of the major proteins photolabeled by native tetracycline and tetracycline photoproducts and implications for the inhibitory action of tetracycline on protein synthesis. Biochemistry. 1983 Jan 18;22(2):359–368. doi: 10.1021/bi00271a020. [DOI] [PubMed] [Google Scholar]
- Hillen W., Gatz C., Altschmied L., Schollmeier K., Meier I. Control of expression of the Tn10-encoded tetracycline resistance genes. Equilibrium and kinetic investigation of the regulatory reactions. J Mol Biol. 1983 Sep 25;169(3):707–721. doi: 10.1016/s0022-2836(83)80166-1. [DOI] [PubMed] [Google Scholar]
- Hillen W., Klock G., Kaffenberger I., Wray L. V., Reznikoff W. S. Purification of the TET repressor and TET operator from the transposon Tn10 and characterization of their interaction. J Biol Chem. 1982 Jun 10;257(11):6605–6613. [PubMed] [Google Scholar]
- Hillen W., Schollmeier K., Gatz C. Control of expression of the Tn10-encoded tetracycline resistance operon. II. Interaction of RNA polymerase and TET repressor with the tet operon regulatory region. J Mol Biol. 1984 Jan 15;172(2):185–201. doi: 10.1016/s0022-2836(84)80037-6. [DOI] [PubMed] [Google Scholar]
- Horaud T., Delbos F., Pepper K. Does a tetracycline resistance determinant of class N exist? Antimicrob Agents Chemother. 1990 Jul;34(7):1447–1449. doi: 10.1128/aac.34.7.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hächler H., Cohen S. P., Levy S. B. marA, a regulated locus which controls expression of chromosomal multiple antibiotic resistance in Escherichia coli. J Bacteriol. 1991 Sep;173(17):5532–5538. doi: 10.1128/jb.173.17.5532-5538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hächler H., Kayser F. H., Berger-Bächi B. Homology of a transferable tetracycline resistance determinant of Clostridium difficile with Streptococcus (Enterococcus) faecalis transposon Tn916. Antimicrob Agents Chemother. 1987 Jul;31(7):1033–1038. doi: 10.1128/aac.31.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives C. L., Bott K. F. Analysis of the tet gene of plasmid pCIS7 isolated from Bacillus subtilis. Gene. 1990 Sep 28;94(1):115–119. doi: 10.1016/0378-1119(90)90476-8. [DOI] [PubMed] [Google Scholar]
- Ives C. L., Bott K. F. Cloned Bacillus subtilis chromosomal DNA mediates tetracycline resistance when present in multiple copies. J Bacteriol. 1989 Apr;171(4):1801–1810. doi: 10.1128/jb.171.4.1801-1810.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. R., Morse S. A. Antibiotic resistance in Neisseria gonorrhoeae: genetics and mechanisms of resistance. Sex Transm Dis. 1988 Oct-Dec;15(4):217–224. doi: 10.1097/00007435-198810000-00008. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Doern G. V., Maher L. A., Howell A. W., Redding J. S. Antimicrobial resistance among respiratory isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in the United States. Antimicrob Agents Chemother. 1990 Nov;34(11):2075–2080. doi: 10.1128/aac.34.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar S. K., Edlind T. D. Enhanced antiparasitic activity of lipophilic tetracyclines: role of uptake. Antimicrob Agents Chemother. 1991 Nov;35(11):2198–2202. doi: 10.1128/aac.35.11.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A., Novick R. P. Complete nucleotide sequence of pT181, a tetracycline-resistance plasmid from Staphylococcus aureus. Plasmid. 1983 Nov;10(3):251–259. doi: 10.1016/0147-619x(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Klock G., Unger B., Gatz C., Hillen W., Altenbuchner J., Schmid K., Schmitt R. Heterologous repressor-operator recognition among four classes of tetracycline resistance determinants. J Bacteriol. 1985 Jan;161(1):326–332. doi: 10.1128/jb.161.1.326-332.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Johnson S. R., Zenilman J. M., Roberts M. C., Morse S. A. High-level tetracycline resistance resulting from TetM in strains of Neisseria spp., Kingella denitrificans, and Eikenella corrodens. Antimicrob Agents Chemother. 1988 May;32(5):765–767. doi: 10.1128/aac.32.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. A., Lopez P., Greenberg B., Espinosa M. Identification and analysis of genes for tetracycline resistance and replication functions in the broad-host-range plasmid pLS1. J Mol Biol. 1986 Dec 20;192(4):753–765. doi: 10.1016/0022-2836(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Le Bouguénec C., de Cespédès G., Horaud T. Presence of chromosomal elements resembling the composite structure Tn3701 in streptococci. J Bacteriol. 1990 Feb;172(2):727–734. doi: 10.1128/jb.172.2.727-734.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N., Titmas B. M., Smith C. J., Tenover F. C. Nucleotide sequence analysis of tetracycline resistance gene tetO from Streptococcus mutans DL5. J Bacteriol. 1988 Aug;170(8):3618–3626. doi: 10.1128/jb.170.8.3618-3626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. M., Burdett V., Courvalin P., Hillen W., Roberts M. C., Taylor D. E. Nomenclature for tetracycline resistance determinants. Antimicrob Agents Chemother. 1989 Aug;33(8):1373–1374. doi: 10.1128/aac.33.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- Levy S. B. Starting life resistance-free. N Engl J Med. 1990 Aug 2;323(5):335–337. doi: 10.1056/NEJM199008023230509. [DOI] [PubMed] [Google Scholar]
- Manavathu E. K., Fernandez C. L., Cooperman B. S., Taylor D. E. Molecular studies on the mechanism of tetracycline resistance mediated by Tet(O). Antimicrob Agents Chemother. 1990 Jan;34(1):71–77. doi: 10.1128/aac.34.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B., Morrissey S., Flynn P., Levy S. B. A new tetracycline-resistance determinant, class E, isolated from Enterobacteriaceae. Gene. 1986;50(1-3):111–117. doi: 10.1016/0378-1119(86)90315-x. [DOI] [PubMed] [Google Scholar]
- Marshall B., Tachibana C., Levy S. B. Frequency of tetracycline resistance determinant classes among lactose-fermenting coliforms. Antimicrob Agents Chemother. 1983 Dec;24(6):835–840. doi: 10.1128/aac.24.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Park B. H., Burdett V., Levy S. B. Energy-dependent efflux mediated by class L (tetL) tetracycline resistance determinant from streptococci. Antimicrob Agents Chemother. 1987 Oct;31(10):1648–1650. doi: 10.1128/aac.31.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Mikulík K., Jiránová A., Janda I., Weiser J. Susceptibility of ribosomes of the tetracycline-producing strain of Streptomyces aureofaciens to tetracyclines. FEBS Lett. 1983 Feb 7;152(1):125–130. doi: 10.1016/0014-5793(83)80496-7. [DOI] [PubMed] [Google Scholar]
- Moran J. S., Zenilman J. M. Therapy for gonococcal infections: options in 1989. Rev Infect Dis. 1990 Jul-Aug;12 (Suppl 6):S633–S644. doi: 10.1093/clinids/12.supplement_6.s633. [DOI] [PubMed] [Google Scholar]
- Mullany P., Wilks M., Lamb I., Clayton C., Wren B., Tabaqchali S. Genetic analysis of a tetracycline resistance element from Clostridium difficile and its conjugal transfer to and from Bacillus subtilis. J Gen Microbiol. 1990 Jul;136(7):1343–1349. doi: 10.1099/00221287-136-7-1343. [DOI] [PubMed] [Google Scholar]
- Naglich J. G., Andrews R. E., Jr Tn916-dependent conjugal transfer of PC194 and PUB110 from Bacillus subtilis into Bacillus thuringiensis subsp. israelensis. Plasmid. 1988 Sep;20(2):113–126. doi: 10.1016/0147-619x(88)90014-5. [DOI] [PubMed] [Google Scholar]
- Nesin M., Svec P., Lupski J. R., Godson G. N., Kreiswirth B., Kornblum J., Projan S. J. Cloning and nucleotide sequence of a chromosomally encoded tetracycline resistance determinant, tetA(M), from a pathogenic, methicillin-resistant strain of Staphylococcus aureus. Antimicrob Agents Chemother. 1990 Nov;34(11):2273–2276. doi: 10.1128/aac.34.11.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich M. P., Shoemaker N. B., Salyers A. A. A Bacteroides tetracycline resistance gene represents a new class of ribosome protection tetracycline resistance. Antimicrob Agents Chemother. 1992 May;36(5):1005–1012. doi: 10.1128/aac.36.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Scott J. R. The presence of conjugative transposon Tn916 in the recipient strain does not impede transfer of a second copy of the element. J Bacteriol. 1991 Jan;173(1):319–324. doi: 10.1128/jb.173.1.319-324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. F. Resistance of bacteria to antibacterial agents: report of Task Force 2. Rev Infect Dis. 1987 May-Jun;9 (Suppl 3):S244–S260. doi: 10.1093/clinids/9.supplement_3.s244. [DOI] [PubMed] [Google Scholar]
- Ohnuki T., Katoh T., Imanaka T., Aiba S. Molecular cloning of tetracycline resistance genes from Streptomyces rimosus in Streptomyces griseus and characterization of the cloned genes. J Bacteriol. 1985 Mar;161(3):1010–1016. doi: 10.1128/jb.161.3.1010-1016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva B., Chopra I. Tet determinants provide poor protection against some tetracyclines: further evidence for division of tetracyclines into two classes. Antimicrob Agents Chemother. 1992 Apr;36(4):876–878. doi: 10.1128/aac.36.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. H., Hendricks M., Malamy M. H., Tally F. P., Levy S. B. Cryptic tetracycline resistance determinant (class F) from Bacteroides fragilis mediates resistance in Escherichia coli by actively reducing tetracycline accumulation. Antimicrob Agents Chemother. 1987 Nov;31(11):1739–1743. doi: 10.1128/aac.31.11.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart-Salmeron C., Trieu-Cuot P., Carlier C., Courvalin P. The integration-excision system of the conjugative transposon Tn 1545 is structurally and functionally related to those of lambdoid phages. Mol Microbiol. 1990 Sep;4(9):1513–1521. doi: 10.1111/j.1365-2958.1990.tb02062.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen B., Noller H. F., Daubresse G., Oliva B., Misulovin Z., Rothstein D. M., Ellestad G. A., Gluzman Y., Tally F. P., Chopra I. Molecular basis of tetracycline action: identification of analogs whose primary target is not the bacterial ribosome. Antimicrob Agents Chemother. 1991 Nov;35(11):2306–2311. doi: 10.1128/aac.35.11.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C. Characterization of the Tet M determinants in urogenital and respiratory bacteria. Antimicrob Agents Chemother. 1990 Mar;34(3):476–478. doi: 10.1128/aac.34.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Hillier S. L. Genetic basis of tetracycline resistance in urogenital bacteria. Antimicrob Agents Chemother. 1990 Feb;34(2):261–264. doi: 10.1128/aac.34.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Knapp J. S. Host range of the conjugative 25.2-megadalton tetracycline resistance plasmid from Neisseria gonorrhoeae and related species. Antimicrob Agents Chemother. 1988 Apr;32(4):488–491. doi: 10.1128/aac.32.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Pang Y. J., Spencer R. C., Winstanley T. G., Brown B. A., Wallace R. J., Jr Tetracycline resistance in Moraxella (Branhamella) catarrhalis: demonstration of two clonal outbreaks by using pulsed-field gel electrophoresis. Antimicrob Agents Chemother. 1991 Nov;35(11):2453–2455. doi: 10.1128/aac.35.11.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C. Tetracycline resistance in Peptostreptococcus species. Antimicrob Agents Chemother. 1991 Aug;35(8):1682–1684. doi: 10.1128/aac.35.8.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouch D. A., Cram D. S., DiBerardino D., Littlejohn T. G., Skurray R. A. Efflux-mediated antiseptic resistance gene qacA from Staphylococcus aureus: common ancestry with tetracycline- and sugar-transport proteins. Mol Microbiol. 1990 Dec;4(12):2051–2062. doi: 10.1111/j.1365-2958.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., Speer B. S., Shoemaker N. B. New perspectives in tetracycline resistance. Mol Microbiol. 1990 Jan;4(1):151–156. doi: 10.1111/j.1365-2958.1990.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Brown J. T., Roberts M., Urdea M. S. Homology of the TetM with translational elongation factors: implications for potential modes of tetM-conferred tetracycline resistance. Nucleic Acids Res. 1988 Feb 11;16(3):1218–1218. doi: 10.1093/nar/16.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Hiruma R., Kawana N., Kaneko M., Taniyasu F., Inami A. Outer membrane permeation of beta-lactam antibiotics in Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. Antimicrob Agents Chemother. 1982 Oct;22(4):585–592. doi: 10.1128/aac.22.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senghas E., Jones J. M., Yamamoto M., Gawron-Burke C., Clewell D. B. Genetic organization of the bacterial conjugative transposon Tn916. J Bacteriol. 1988 Jan;170(1):245–249. doi: 10.1128/jb.170.1.245-249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan R. P., Chopra I. Origin of tetracycline efflux proteins: conclusions from nucleotide sequence analysis. Mol Microbiol. 1991 Apr;5(4):895–900. doi: 10.1111/j.1365-2958.1991.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Barber R. D., Salyers A. A. Cloning and characterization of a Bacteroides conjugal tetracycline-erythromycin resistance element by using a shuttle cosmid vector. J Bacteriol. 1989 Mar;171(3):1294–1302. doi: 10.1128/jb.171.3.1294-1302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Wang G. R., Salyers A. A. Evidence for natural transfer of a tetracycline resistance gene between bacteria from the human colon and bacteria from the bovine rumen. Appl Environ Microbiol. 1992 Apr;58(4):1313–1320. doi: 10.1128/aem.58.4.1313-1320.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Rams T. E. Antibiotics in periodontal therapy: advantages and disadvantages. J Clin Periodontol. 1990 Aug;17(7 ):479–493. doi: 10.1111/j.1365-2710.1992.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Speer B. S., Bedzyk L., Salyers A. A. Evidence that a novel tetracycline resistance gene found on two Bacteroides transposons encodes an NADP-requiring oxidoreductase. J Bacteriol. 1991 Jan;173(1):176–183. doi: 10.1128/jb.173.1.176-183.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer B. S., Salyers A. A. A tetracycline efflux gene on Bacteroides transposon Tn4400 does not contribute to tetracycline resistance. J Bacteriol. 1990 Jan;172(1):292–298. doi: 10.1128/jb.172.1.292-298.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer B. S., Salyers A. A. Characterization of a novel tetracycline resistance that functions only in aerobically grown Escherichia coli. J Bacteriol. 1988 Apr;170(4):1423–1429. doi: 10.1128/jb.170.4.1423-1429.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer B. S., Salyers A. A. Novel aerobic tetracycline resistance gene that chemically modifies tetracycline. J Bacteriol. 1989 Jan;171(1):148–153. doi: 10.1128/jb.171.1.148-153.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spika J. S., Waterman S. H., Hoo G. W., St Louis M. E., Pacer R. E., James S. M., Bissett M. L., Mayer L. W., Chiu J. Y., Hall B. Chloramphenicol-resistant Salmonella newport traced through hamburger to dairy farms. A major persisting source of human salmonellosis in California. N Engl J Med. 1987 Mar 5;316(10):565–570. doi: 10.1056/NEJM198703053161001. [DOI] [PubMed] [Google Scholar]
- Stevens A. M., Sanders J. M., Shoemaker N. B., Salyers A. A. Genes involved in production of plasmidlike forms by a Bacteroides conjugal chromosomal element share amino acid homology with two-component regulatory systems. J Bacteriol. 1992 May;174(9):2935–2942. doi: 10.1128/jb.174.9.2935-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. M., Shoemaker N. B., Salyers A. A. The region of a Bacteroides conjugal chromosomal tetracycline resistance element which is responsible for production of plasmidlike forms from unlinked chromosomal DNA might also be involved in transfer of the element. J Bacteriol. 1990 Aug;172(8):4271–4279. doi: 10.1128/jb.172.8.4271-4279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y. A., He P., Clewell D. B. Characterization of the tet(M) determinant of Tn916: evidence for regulation by transcription attenuation. Antimicrob Agents Chemother. 1992 Apr;36(4):769–778. doi: 10.1128/aac.36.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Altschmied L., Hillen W. Kinetic and equilibrium characterization of the Tet repressor-tetracycline complex by fluorescence measurements. Evidence for divalent metal ion requirement and energy transfer. J Mol Biol. 1986 Feb 5;187(3):341–348. doi: 10.1016/0022-2836(86)90437-7. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Courvalin P. Mechanisms of antibiotic resistance in Campylobacter species. Antimicrob Agents Chemother. 1988 Aug;32(8):1107–1112. doi: 10.1128/aac.32.8.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokue Y., Shoji S., Satoh K., Watanabe A., Motomiya M. Comparison of a polymerase chain reaction assay and a conventional microbiologic method for detection of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1992 Jan;36(1):6–9. doi: 10.1128/aac.36.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey K. E., Barnes R. C. Treatment of Chlamydia trachomatis genital infection. Rev Infect Dis. 1990 Jul-Aug;12 (Suppl 6):S645–S655. doi: 10.1093/clinids/12.supplement_6.s645. [DOI] [PubMed] [Google Scholar]
- Torres O. R., Korman R. Z., Zahler S. A., Dunny G. M. The conjugative transposon Tn925: enhancement of conjugal transfer by tetracycline in Enterococcus faecalis and mobilization of chromosomal genes in Bacillus subtilis and E. faecalis. Mol Gen Genet. 1991 Mar;225(3):395–400. doi: 10.1007/BF00261679. [DOI] [PubMed] [Google Scholar]
- Tovar K., Ernst A., Hillen W. Identification and nucleotide sequence of the class E tet regulatory elements and operator and inducer binding of the encoded purified Tet repressor. Mol Gen Genet. 1988 Dec;215(1):76–80. doi: 10.1007/BF00331306. [DOI] [PubMed] [Google Scholar]
- Vining L. C. Antibiotic tolerance in producer organisms. Adv Appl Microbiol. 1979;25:147–168. doi: 10.1016/s0065-2164(08)70149-7. [DOI] [PubMed] [Google Scholar]
- Wang Y., Taylor D. E. A DNA sequence upstream of the tet(O) gene is required for full expression of tetracycline resistance. Antimicrob Agents Chemother. 1991 Oct;35(10):2020–2025. doi: 10.1128/aac.35.10.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Ohmori H., Kaneko-Ohdera M., Nomura T., Sawai T. Delta pH-dependent accumulation of tetracycline in Escherichia coli. Antimicrob Agents Chemother. 1991 Jan;35(1):53–56. doi: 10.1128/aac.35.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilhao R., Papadopoulou B., Courvalin P. Occurrence of the Campylobacter resistance gene tetO in Enterococcus and Streptococcus spp. Antimicrob Agents Chemother. 1988 Dec;32(12):1793–1796. doi: 10.1128/aac.32.12.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barbeyrac B., Dutilh B., Quentin C., Renaudin H., Bébéar C. Susceptibility of Bacteroides ureolyticus to antimicrobial agents and identification of a tetracycline resistance determinant related to tetM. J Antimicrob Chemother. 1991 Jun;27(6):721–731. doi: 10.1093/jac/27.6.721. [DOI] [PubMed] [Google Scholar]



