Abstract
Probe-based confocal laser endomicroscopy (CLE) is an emerging optical imaging technology that enables real-time in vivo microscopy of mucosal surfaces during standard endoscopy. With applications currently in the respiratory1 and gastrointestinal tracts,2-6 CLE has also been explored in the urinary tract for bladder cancer diagnosis.7-10 Cellular morphology and tissue microarchitecture can be resolved with micron scale resolution in real time, in addition to dynamic imaging of the normal and pathological vasculature.7
The probe-based CLE system (Cellvizio, Mauna Kea Technologies, France) consists of a reusable fiberoptic imaging probe coupled to a 488 nm laser scanning unit. The imaging probe is inserted in the working channels of standard flexible and rigid endoscopes. An endoscope-based CLE system (Optiscan, Australia), in which the confocal endomicroscopy functionality is integrated onto the endoscope, is also used in the gastrointestinal tract. Given the larger scope diameter, however, application in the urinary tract is currently limited to ex vivo use.11 Confocal image acquisition is done through direct contact of the imaging probe with the target tissue and recorded as video sequences. As in the gastrointestinal tract, endomicroscopy of the urinary tract requires an exogenenous contrast agent—most commonly fluorescein, which can be administered intravenously or intravesically. Intravesical administration is a well-established method to introduce pharmacological agents locally with minimal systemic toxicity that is unique to the urinary tract. Fluorescein rapidly stains the extracellular matrix and has an established safety profile.12 Imaging probes of various diameters enable compatibility with different caliber endoscopes. To date, 1.4 and 2.6 mm probes have been evaluated with flexible and rigid cystoscopy.10 Recent availability of a < 1 mm imaging probe13 opens up the possibility of CLE in the upper urinary tract during ureteroscopy. Fluorescence cystoscopy (i.e. photodynamic diagnosis) and narrow band imaging are additional endoscope-based optical imaging modalities14 that can be combined with CLE to achieve multimodal imaging of the urinary tract. In the future, CLE may be coupled with molecular contrast agents such as fluorescently labeled peptides15 and antibodies for endoscopic imaging of disease processes with molecular specificity.
Keywords: Medicine, Issue 71, Anatomy, Physiology, Cancer Biology, Surgery, Basic Protocols, Confocal laser endomicroscopy, microscopy, endoscopy, cystoscopy, human bladder, bladder cancer, urology, minimally invasive, cellular imaging
Protocol
1. Patient Preparation
Consent patient scheduled for diagnostic cystoscopy and other endourological procedures such as transurethral resection of bladder tumor (TURBT) for CLE. Include in the consent a description of the use of intravesical and/or intravenous fluorescein as the contrast agent. Inquire history of hypersensitivity reaction to fluorescein.
Patient is positioned for cystoscopy (typically in lithotomy position) and prepared in a sterile fashion.
Proceed with standard white light cystoscopy (WLC) with a rigid or flexible cystoscope through the urethra.
Survey all regions of the bladder under white light (Figure 1) and note regions of interest for CLE investigation.
In addition to WLC, the bladder may be imaged with other macroscopic optical imaging modalities including fluorescence cystoscopy (Figure 2A) or narrow band imaging (Figure 2B) to identify additional areas of suspicion followed by characterization with CLE.
Use a cautery electrode or resection loop to place a small cautery mark adjacent to the regions of interest that will both be imaged and biopsied for pathological confirmation. The cautery mark facilitates re-localization for subsequent CLE imaging and biopsy.
2. Contrast Agent
- Intravesical instillation of contrast agent
- Prepare by diluting clinical grade 10% fluorescein sodium in sterile normal saline (0.9% NaCl) to desired concentration. For example, prepare 400 ml of 0.1% fluorescein by diluting 4 ml of 10% fluorescein in 396 ml of normal saline.
- Insert a sterile urinary catheter into the bladder and instill diluted contrast agent by gravity using a 60 ml catheter-tip syringe.
- Clamp the urinary catheter to hold contrast agent indwelling for 5 min.
- Drain contrast agent from bladder. Use the 60 ml catheter-tip syringe to wash out residual contrast from the bladder using normal saline, about 240-300 ml.
- Proceed to CLE imaging.
- Intravenous injection of fluorescein
- Draw 1.0 ml of fluorescein into syringe. Attach syringe to intravenous access and inject 0.5 - 1.0 ml of fluorescein intravenously as a bolus. Flush line with normal saline. This is typically done by the anesthesia team in the operating room.
- Proceed to CLE imaging.
3. CLE Imaging
Remove the sterilized CLE imaging probe (Cellvizio) from its packaging and connect it to the laser scanning unit (Mauna Kea Technologies, Paris, France)
Warm up and calibrate the imaging probe by following the manufacturer's instructions.
Re-insert the cystoscope or resectoscope into the bladder. Use a 0 degree lens with the rigid cystoscope.
Advance the CLE imaging probe along the working channel of the cystoscope with the tip just beyond the cystoscope.
Under white light, locate the previously marked regions of interest. Use additional normal saline irrigation as needed for visualization of the mucosa and maintain moderate distension of the bladder.
- Manipulate CLE imaging probe with the operator's hand for direct en face contact of the regions of interest. For optical sectioning of the regions of interest, gently increase and decrease pressure to the imaging probe while maintaining direct contact.
- To reach tumors located at the anterior bladder, a standard Albarran bridge may help facilitate deflection of the imaging probe for direct contact with the region of interest (Figure 2C).
Common regions of interest include papillary tumor, non-papillary tumor, transition between normal-appearing mucosa and tumor, and erythematous patches, which can all be imaged using either intravesical or intravenous fluorescein administration. Intravenous fluorescein is required for imaging of the resection bed, prostatic urethra, and penile urethra.
As the imaging probe scans region of interest, record images for future analysis. Note overall cellular morphology, boundary, organization, and presence of neo-angiogenesis.
Upon completion of imaging, remove CLE imaging probe from working channel.
If desired, obtain cold-cup biopsies from corresponding regions of interest that were scanned by CLE. Use cautery markings as landmarks for co-registration of the imaged areas that are biopsied.
Complete TURBT per routine. In patients who received intravenous fluorescein, consider using continuous flow resectoscopes to improve visualization during resection, as fluorescein is excreted by the kidney into the bladder approximately 5 min after intravenous administration.
Retrieve saved image recordings from confocal processor and conduct image analysis.
Representative Results
CLE images are saved as grayscale video sequence files at 12 frames per second. Image interpretation is done in real time and may be used to impact clinical decision making under an investigational protocol. Offline analysis, which includes reviewing of the video sequence, additional image processing such as mosaicing,7 and comparison with standard pathology, are important during the learning curve phase associated with the technology. Figures 1B and 1C are representative CLE images obtained from two different bladder tumors using intravesical and intravenous fluorescein, respectively. For bladder tumors, cellular features (e.g. morphology, cohesiveness and borders) and microarchitectural features (e.g. flat versus papillary tumors, tissue organization and vascular features) are noted to differentiate between benign and malignant lesions and low and high grade cancer. Given the imaging probes may be interchanged between different types of cystoscopes, CLE can be combined with other emerging imaging modalities including fluorescence cystoscopy (Figure 2A) and narrow band imaging (Figure 2B), which may improve the diagnostic accuracy of either technology by itself.
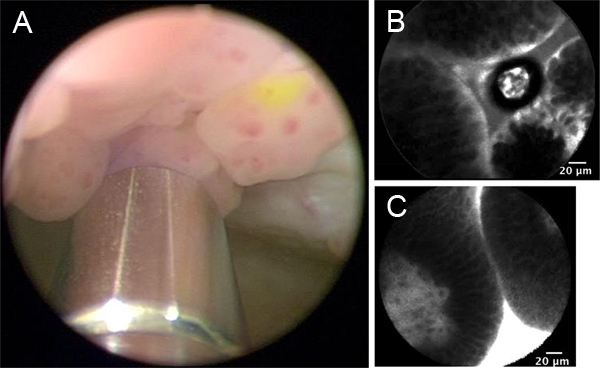 Figure 1. Representative images from WLC and CLE dependent on intravesical versus intravenous administration of fluorescein. A. WLC with intravesically stained papillary tumor. 2.6-mm imaging probe is visible at the bottom of the image. B. CLE of intravesically stained papillary tumor. C. CLE of intravenously stained papillary tumor. Click here to view larger figure.
Figure 1. Representative images from WLC and CLE dependent on intravesical versus intravenous administration of fluorescein. A. WLC with intravesically stained papillary tumor. 2.6-mm imaging probe is visible at the bottom of the image. B. CLE of intravesically stained papillary tumor. C. CLE of intravenously stained papillary tumor. Click here to view larger figure.
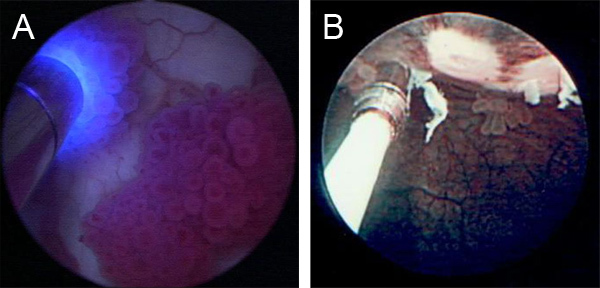 Figure 2. Adjunctive macroscopic imaging modalities and additional tools. A. CLE imaging in conjunction with fluorescence cystoscopy (with administration of hexaminolevulinate hydrochloride). CLE imaging probe is visible on left side of image. B. CLE imaging in conjunction with narrow band imaging. CLE imaging probe is visible on left side of image.
Figure 2. Adjunctive macroscopic imaging modalities and additional tools. A. CLE imaging in conjunction with fluorescence cystoscopy (with administration of hexaminolevulinate hydrochloride). CLE imaging probe is visible on left side of image. B. CLE imaging in conjunction with narrow band imaging. CLE imaging probe is visible on left side of image.
Discussion
Attaining and maintaining solid en face contact between the imaging probe and the bladder mucosa is the most critical step in acquiring optimal image quality. There is approximately a 3-5 patient learning curve to develop the dexterity to manipulate the imaging probe and to hold the probe steady during image acquisition. Additionally, as this procedure is conducted in vivo, patient movements (i.e. respiratory) and vascular pulsations may affect the imaging probe contact with the bladder. The interobserver variance and the learning curve of image interpretation, which requires experience with wide spectrum of bladder pathology, are currently under investigation. Variation in image quality may be attributed to tissue variability and underlying pathology. Imaging of the normal areas, in addition to the suspicious appearing areas, is useful to assess image quality and fluorescein staining.
Cautery marking of each region of interest is helpful in facilitating re-localization of the imaged and excised tissues, as the bladder is emptied and refilled repeatedly between image acquisition and resection. This step, called co-registration, is important for comparative analysis to correlate images obtained by CLE with histology. Both procedures for intravesical and intravenous administration of fluorescein are described above, with some differences in regards to timing and requirement for intravenous access.8
Depending on the anatomic location and microscopic features to be imaged, the optimal mode and timing of the fluorescein administration will need to be determined experimentally. In patients who received intravesical fluorescein, additional intravenous fluorescein administration may be given to image the regions that were not stained (e.g. tumor bed, urethra).
A 2.6 mm imaging probe was used in this demonstration. However, the principle of accessing the bladder with probe-based technology could be utilized in future generations of imaging probes that enhance image quality in smaller and more maneuverable probes. Imaging probes 1 mm in diameter have recently been described.13 These smaller imaging probes may allow CLE imaging in outpatient cystoscopy as well as in vivo confocal imaging of the upper urinary tract during ureteroscopy.
Finally, novel contrast agents specific to bladder cancer may be developed that improves image quality. The technique demonstrated of instilling contrast agents intravesically holds significant promise since the patient has lower risk of systemic exposure compared to intravenous injection.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors would like to thank Mauna Kea Technologies for technical support. The authors also thank Shelly Hsiao for technical assistance and Kathleen E. Mach for critical review. This work was supported in part by NIH R01CA160986 to J.C.L.
References
- Thiberville L, et al. Human in vivo fluorescence microimaging of the alveolar ducts and sacs during bronchoscopy. Eur. Respir. J. 2009;33:974–985. doi: 10.1183/09031936.00083708. [DOI] [PubMed] [Google Scholar]
- Dunbar KB, Okolo P, 3rd, Montgomery E, Canto MI. Confocal laser endomicroscopy in Barrett's esophagus and endoscopically inapparent Barrett's neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. Gastrointest. Endosc. 2009;70:645–654. doi: 10.1016/j.gie.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner AM, et al. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology. 2010;138:834–842. doi: 10.1053/j.gastro.2009.10.053. [DOI] [PubMed] [Google Scholar]
- Pech O, et al. Confocal laser endomicroscopy for in vivo diagnosis of early squamous cell carcinoma in the esophagus. Clin. Gastroenterol. Hepatol. 2008;6:89–94. doi: 10.1016/j.cgh.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Goetz M, et al. In vivo confocal laser endo microscopy of the human liver: a novel method for assessing liver microarchitecture in real time. Endoscopy. 2008;40:554–562. doi: 10.1055/s-2008-1077296. [DOI] [PubMed] [Google Scholar]
- Meining A, et al. Detection of cholangiocarcinoma in vivo using miniprobe-based confocal fluorescence microscopy. Clin. Gastroenterol. Hepatol. 2008;6:1057–1060. doi: 10.1016/j.cgh.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Wu K, et al. Dynamic real-time microscopy of the urinary tract using confocal laser endomicroscopy. Urology. 2011;78:225–231. doi: 10.1016/j.urology.2011.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonn GA, et al. Optical biopsy of human bladder neoplasia with in vivo confocal laser endomicroscopy. J. Urol. 2009;182:1299–1305. doi: 10.1016/j.juro.2009.06.039. [DOI] [PubMed] [Google Scholar]
- Sonn GA, et al. Fibered confocal microscopy of bladder tumors: an ex vivo study. J. Endourol. 2009;23:197–201. doi: 10.1089/end.2008.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams W, et al. Comparison of 2.6- and 1.4-mm imaging probes for confocal laser endomicroscopy of the urinary tract. J. Endourol. 2011;25:917–921. doi: 10.1089/end.2010.0686. [DOI] [PubMed] [Google Scholar]
- Wiesner C, et al. Confocal laser endomicroscopy for the diagnosis of urothelial bladder neoplasia: a technology of the future? BJU Int. 2011;107:399–403. doi: 10.1111/j.1464-410X.2010.09540.x. [DOI] [PubMed] [Google Scholar]
- Wallace MB, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2010;31:548–552. doi: 10.1111/j.1365-2036.2009.04207.x. [DOI] [PubMed] [Google Scholar]
- Konda VJA, et al. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos. Gastrointest. Endosc. 2011;74:1049–1060. doi: 10.1016/j.gie.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Liu J-J, Droller M, Liao JC. New Optical Imaging Technologies in Bladder Cancer: Considerations and Perspectives. J. Urol. 2012;188:361–368. doi: 10.1016/j.juro.2012.03.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung P-L, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat. Med. 2008;14:454–458. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]


