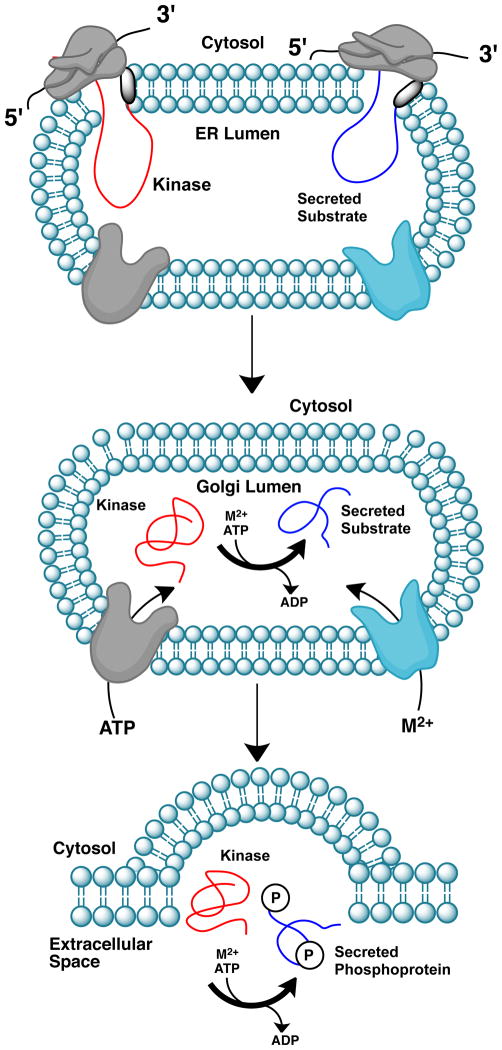
Figure I. Mechanism for the phosphorylation of extracellular proteins by secreted kinases.
Proteins destined for secretion have a short sequence of hydrophobic amino acids at the N-terminus known as the signal peptide (SP, shaded black). During translation, the SPs of the kinase (red) and substrate (blue) bind the signal recognition particle embedded in the endoplasmic reticulum (ER) membrane and the nascent polypeptides enter the lumen of the ER. The signal peptidase subsequently cleaves the SP releasing the proteins from the membrane. ATP and divalent cations (M2+) are transported into the Golgi lumen by specific membrane bound transporters. Catalysis may occur within the Golgi lumen or in the extracellular space.