Abstract
Circadian and ultradian variations of basal glucocorticoid secretion and transient elevations during stress are essential for homeostasis. Using intronic qRT-PCR to measure changes in primary transcript (hnRNA) we have shown that secretory events induced by stress or ACTH injection are followed by episodic increases in transcription of rate limiting steroidogenic proteins, such as steroidogenic acute regulatory protein (StAR), cytochrome P450 side chain cleavage and melanocortin receptor associated protein. These transcriptional episodes imply rapid turnover of steroidogenic proteins and the need of de novo synthesis following each secretory event. In addition to episodic ACTH secretion, it is likely that intracellular feedback mechanisms at the adrenal fasciculata level contribute to the generation of episodes of transcription. The time relationship between activation and translocation of the CREB co-activator, transducer of regulated CREB activity (TORC) to the nucleus preceding transcriptional episodes suggest the involvement of TORC in the transcriptional activation of StAR and other steroidogenic proteins.
Introduction
Glucocorticoids produced in the zona fasciculata of the adrenal gland are the final product of activation of hypothalamic pituitary adrenal (HPA) axis and are essential for normal metabolic activity and adaptation during stress (McEwen, 1998; Munck and Naray-Fejes-Toth, 1994). Their synthesis is initiated by the transport of cholesterol to the inner mitochondrial membrane by steroidogenic acute regulatory protein (StAR) (Stocco and Clark, 1996). This is followed by the conversion of the 27-carbon, cholesterol to the 21-carbon, pregnenolone, by cytochrome 450 11A (CYP 11A) or side chain cleavage enzyme (Churchill and Kimura, 1979). A series of consecutive steps catalyzed by 3β-dehydrogenase/Δ 5–4 isomerase, 21βhydroxylase (or CYP212A), and 11β-hydroxylase (CYP11B1) leads to the formation of glucocorticoids, corticosterone in rat and mouse, and the 17-hydroxylated cortisol in humans and other mammalian species (Bush, 1953; Hum and Miller, 1993). The secretion of glucocorticoids is regulated by pituitary adrenocorticotropic hormone (ACTH) which is under the control of hypothalamic corticotrophin releasing hormone (CRH) (Vale et al., 1981). Adrenal steroidogenesis is activated by the binding of adrenocorticotropin hormone (ACTH) to receptors in the cell surface of steroidogenic cells in the adrenal zona fasciculata, the melanocortin 2 receptor (MC2R), a guanyl nucleotide coupled receptor linked to adenylate cyclase-protein kinase A (PKA) dependent pathways (Mountjoy et al., 1992). In contrast to other G protein coupled receptors, the MC2R requires an accessory protein, called melanocortin receptor accessory protein (MRAP), for its transport to the plasma membrane and biological activity (Metherell et al., 2005).
Glucocorticoids are essential for homeostasis and for survival in severe stress (McEwen, 1998; Munck et al., 1984). In addition, they exert negative feedback in the brain and anterior pituitary corticotrophs to inhibit CRH and ACTH secretion and prevent the deleterious effects of excessive HPA axis activation (Dallman et al., 1994). In basal conditions circulating glucocorticoid levels follow circadian (daily) and ultradian (hourly) rhythms, with levels being low during the resting period, and starting to rise approximately 2 hours before the active period (day time in humans and night time in mice and rats (Desir et al., 1980; Gallagher et al., 1973). The ultradian rhythm consists of hourly pulses throughout the 24h period, with amplitude and frequency varying depending on the circadian phase (Carnes et al., 1989; Lightman et al., 2008). Super-imposed on the circadian and ultradian variations, stress induces rapid and transient increases in blood glucocorticoids, the magnitude and duration of which depends on the duration and nature of the stressor. This episodic nature of glucocorticoid secretion, with ultradian pulses in basal conditions and super-imposed transient increases during stress is essential for homeostasis, especially during stress adaptation. Alteration of this episodic pattern of secretion, leading to prolonged constant levels of circulating glucocorticoids, can result in neuroendocrine, behavioral and metabolic dysfunction due to changes in glucocorticoid receptors and tissue responsiveness to the steroid (Sarabdjitsingh et al., 2010).
Despite the recognized importance of glucocorticoid pulsatility (Lightman et al., 2008; Stavreva et al., 2009), the mechanisms underlying episodic activity in the adrenal are not completely understood. In contrast to peptide hormones which are released from stored pools, steroid hormones cannot be stored in the cell and are released immediately upon synthesis. Rapid activation of proteins involved in steroidogenesis by phosphorylation plays a role in the immediate initiation of steroidogenesis and glucocorticoid secretion upon stimulation of the adrenal by ACTH (Chaudhary and Stocco, 1991; Mikami and Strott, 1986; Miller and Strauss, 1999; Pon et al., 1986). However, the well recognized requirement of de novo protein synthesis for activation of steroidogenesis, suggests that regulation of steroidogenic proteins at the transcriptional or post-transcriptional levels is involved in pulse generation (Ferguson, 1963; Garren et al., 1965). This article describes studies demonstrating that secretory episodes in vivo are associated with transcriptional pulses for a number of important steroidogenic proteins, and explores molecular mechanisms potentially involved in transcriptional pulsatility in the adrenal. All animal procedures described in the article were performed according to NIH guidelines and approved by the NICHD Animal Care and Use Committee, or University of Bristol Ethical Review Group.
1. Episodic glucocorticoid secretion and steroidogenic enzyme transcription
The recognized requirement of de novo steroid biosynthesis for steroidogenesis suggests that rapid induction of proteins important for steroidogenesis plays a role in determining the secretory pattern. To determine whether steroidogenic episodes are associated with transcription of steroidogenic proteins, we measured changes in primary transcript or heteronuclear RNA (hnRNA) of genes including the rate limiting protein StAR, as well as CYP 11A, and MC2R Accessory Protein (MRAP), following activation of steroidogenesis. Levels of hnRNA were measured in decapsulated adrenals by real time PCR using primers against intronic sequences (Spiga et al., 2011a; Spiga et al., 2011b). Since introns are rapidly spliced out to form mature RNA or messenger RNA (mRNA), changes in hnRNA levels are good indicators of gene transcription (Fremeau et al., 1986; Liu et al., 2008; Young et al., 1986). MC2R was also studied but since in rats the gene does not have any introns (Benson et al., 2005; Cone et al., 1993), only mRNA levels could be measured.
1.1 Acute stress induced steroidogenesis
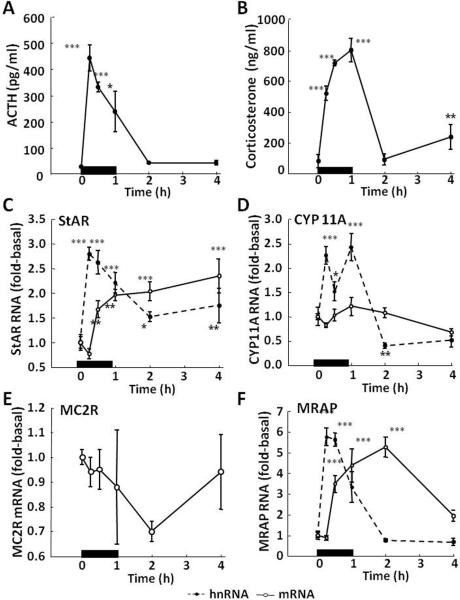
Male Sprage Dawley rats, 250-300 g were subjected to restraint stress for 1 h, and groups were sacrificed by decapitation in control conditions or at the time points indicated in the figures. As shown in Fig 1, the expected increases in plasma ACTH and corticosterone following restraint stress for 1 h (Fig 1-A and B) were accompanied by simultaneous increases in StAR (Fig 1-C) and CYP 11A (Fig 1-D) hnRNA, indicative of increases in transcription. The fact that the glucocorticoid secretion is accompanied by episodes of transcription strongly suggests that the protein undergoes rapid turnover and that de novo protein synthesis is required to sustain adrenal responsiveness. The increases in hnRNA were followed by increases in mRNA, which were marked for StAR but minor and not statistically significant for CYP 11A mRNA. It is well recognized that protein synthesis blockers prevent the initiation of steroidogenesis (Ferguson, 1963) and that activation requires de novo protein synthesis (Garren et al., 1965). This labile protein was later identified as StAR, thus rapid turnover of this rate limiting protein for steroidogenesis was not unexpected. As the first step in steroid biosynthesis; the conversion of cholesterol to pregnenolone catalyzed by CYP 11A or side chain cleavage enzyme is also a rate limiting step. It is noteworthy that this enzyme is also rapidly induced by stress. Whether other enzymes of the steroid biosynthetic pathway are inducible during stress remains to be determined.
Fig 1.
Effects of restraint stress on the levels of ACTH [A] and corticosterone [B] in plasma, and heteronuclear (hn,  ) and messenger (m,
) and messenger (m,  ) RNA levels for StAR [C], cytochrome P450 side chain cleavage (CYP11A) [D], melanocortin type 2 receptor (MC2R) [E] and melanocortin receptor associated protein (MRAP) [F] in the adrenal zona fasciculata. Rats were subjected to restraint stress for 1h (indicated by the black bar) and killed by decapitation at the time points indicated. One-way-ANOVA showed significant effects of stress on plasma hormone levels, and on StAR, CYP 11A and MRAP hnRNAs, and StAR and MRAP mRNAs. MC2R mRNA showed a tendency to decrease. Data points represent the mean and SE of the values in 4 rats per time point. *, p<0.05; **, p<0.01; ***, p<0.001
) RNA levels for StAR [C], cytochrome P450 side chain cleavage (CYP11A) [D], melanocortin type 2 receptor (MC2R) [E] and melanocortin receptor associated protein (MRAP) [F] in the adrenal zona fasciculata. Rats were subjected to restraint stress for 1h (indicated by the black bar) and killed by decapitation at the time points indicated. One-way-ANOVA showed significant effects of stress on plasma hormone levels, and on StAR, CYP 11A and MRAP hnRNAs, and StAR and MRAP mRNAs. MC2R mRNA showed a tendency to decrease. Data points represent the mean and SE of the values in 4 rats per time point. *, p<0.05; **, p<0.01; ***, p<0.001
In addition, stress had marked effects on the expression of proteins related to the adrenal responses to ACTH, the MC2R and the accessory protein MRAP. As seen in Fig 1-E, stress causes a rapid and marked increase in MRAP hnRNA, indicating transcriptional induction by stress. The increases in hnRNA were followed by marked increases in MRAP mRNA. This suggests that regulation of this protein, critical for the biological activity of the MC2R, is important for the control of adrenal sensitivity to ACTH. Since in rodents the MC2R does not have an intron, it was not possible to measure transcription by qPCR. However, in contrast with the marked increases in StAR and MRAP mRNA, stress did not increase MC2R mRNA, but there was a significant decrease by 2 h, followed by recovery to near basal levels by 4 h (Fig 1-F). This is similar to observations with other G-protein coupled receptors, such as the V1b vasopressin receptor and the CRH receptors in which mRNA levels decrease following acute stress (Rabadan-Diehl et al., 1995; Rabadan-Diehl et al., 1996). This suggests that as with the latter receptors, regulation is predominantly at the translational level rather than transcriptional (Aguilera et al., 2003). The decrease in MC2R mRNA could be due to mRNA utilization during translation to newly synthesized protein. In addition, studies in transfected cells have shown that MC2R activation is followed by receptor internalization and recycling to the membrane (Roy et al., 2011). Thus, receptor recycling would decrease the need for transcription for each secretory episode.
1.2 The effect of stress on steroidogenic protein transcription is reproduced by ACTH
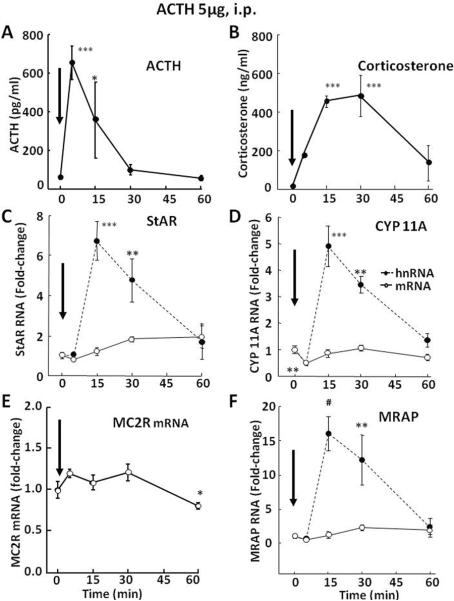
ACTH is the major regulator of steroidogenesis in the adrenal fasciculata and studies in reporter gene assays (Ivell et al., 2000; Manna and Stocco, 2007) or examining changes in mRNA levels (Lehoux et al., 1998; Sewer and Waterman, 2003) suggest that ACTH stimulates StAR transcription. To determine whether ACTH mediates the effect of stress on the transcription of steroidogenic proteins, hnRNA levels for StAR, Cyp 11A, MC2R and MRAP were measured following ACTH injection. As shown in Fig 2-A and B, an injection of synthetic ACTH 1–24, 5 μ-g, i.p. (Cortrosyn, Amphastar Pharmaceuticals, Rancho Cucamonga, CA) in methylprednisolone-suppressed rats (Solu-Medrol, Pharmacia & Upjohn Co, Division of Pfizer Inc, NY, 500 μg/rat, 2 h prior to ACTH injection), increased plasma ACTH and corticosterone in the range of the increases during stress, though of shorter duration (ACTH levels had returned to near basal by 30 min, Fig. 2-A and B). Similar to the observations with restraint stress, elevations in plasma ACTH were followed by marked increases in StAR, CYP 11A and MRAP hnRNA levels, which are in keeping with transcriptional induction. Consistent with the shorter duration of the ACTH increases, hnRNA levels for the 3 proteins returned to basal by 60 min (Fig 2-C, D and F). The changes in mRNA were minor and not statistically significant. Similar to the responses to stress, MC2R mRNA showed a reduction at 1 h, the latest time point measured (Fig 2-E). These data suggest that the effects of restraint stress on steroidogenic protein transcription are mediated by ACTH.
Fig 2.
Effects of ACTH injection, 5μg, i.p., on the levels of ACTH [A] and corticosterone [B] in plasma, and heteronuclear (hn,  ) and messenger (m,
) and messenger (m,  ) RNA levels for StAR [C], cytochrome P450 side chain cleavage (CYP11A) [D], melanocortin type 2 receptor (MC2R) [E] and melanocortin receptor associated protein (MRAP) [F]. Rats were killed by decapitation at the time points indicated. One-way-ANOVA showed significant effects of stress on plasma hormone levels, and on StAR, CYP 11A and MRAP hnRNAs, and StAR and MRAP mRNAs. MC2R mRNA showed a tendency to decrease. Data points represent the mean and SE of the values in 4 rats per time point. *, p<0.05; **, p<0.01; ***, p<0.001. The arrows indicate the time of ACTH injection.
) RNA levels for StAR [C], cytochrome P450 side chain cleavage (CYP11A) [D], melanocortin type 2 receptor (MC2R) [E] and melanocortin receptor associated protein (MRAP) [F]. Rats were killed by decapitation at the time points indicated. One-way-ANOVA showed significant effects of stress on plasma hormone levels, and on StAR, CYP 11A and MRAP hnRNAs, and StAR and MRAP mRNAs. MC2R mRNA showed a tendency to decrease. Data points represent the mean and SE of the values in 4 rats per time point. *, p<0.05; **, p<0.01; ***, p<0.001. The arrows indicate the time of ACTH injection.
1.3 Stimulation of ultradian corticosterone pulses by ACTH induces peaks of transcription
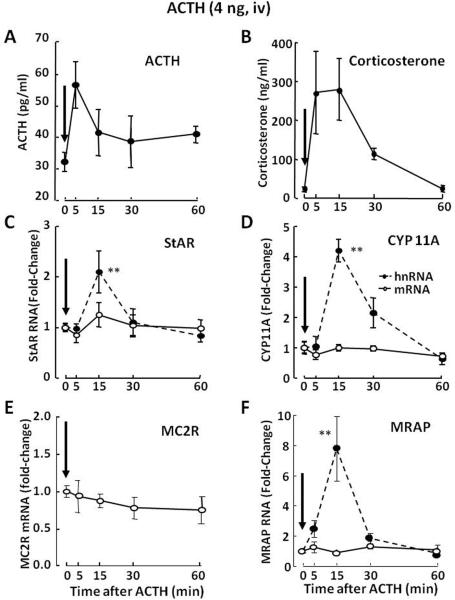
Studies examining the effect of small intravenous ACTH pulses to mimic normal ultradian pulsatility of glucocorticoid secretion have shown that minor increases in plasma ACTH levels are sufficient to induce StAR, CYP11A and MRAP transcription (Spiga et al., 2011a). In these experiments, an i.v. injection of 4ng of ACTH 1–24 (Synacthen, Alliance Pharmaceutical, Chippenham, UK) caused a transient, about 25-fold increase in plasma corticosterone levels, comparable to an ultradian pulse, in spite of non-statistically significant increases in circulating ACTH (Fig 3-A and B). The increases in plasma corticosterone were accompanied by rapid and significant increases in StAR, CYP 11A and MRAP hnRNA levels, 3 min after injection (Fig 3-C, D and F). In contrast to the increases in StAR and MRAP mRNA following stress and higher ACTH doses, RNA levels for these proteins were unchanged following injection of the low ACTH dose. This apparent lack of change is probably due to increased mRNA turnover as the result of protein synthesis. Again, MC2R mRNA levels did not increase but showed a tendency to decrease (Fig 3-E).
Fig 3.
Effects of a low dose ACTH injection (4ng, iv) on the levels of ACTH [A] and corticosterone [B] in plasma, and adrenal zona fasciculata levels of heteronuclear (hn,  ) and messenger (m,
) and messenger (m,  ) RNA levels for StAR [C], cytochrome P450 side chain cleavage (CYP11A) [D], melanocortin type 2 receptor (MC2R) [E] and melanocortin receptor associated protein (MRAP) [F]. Rats were killed by decapitation at the time points indicated. Plasma ACTH levels tended to increase at 5 min. This small and non-statistically significant increase in plasma ACTH injection caused significant increases in plasma corticosterone levels, accompanied by significant increases in StAR, CYP 11A and MRAP hnRNAs, and StAR and MRAP mRNAs. No changes in MC2R mRNA were observed following the ACTH injection. Data points represent the mean and SE of the values in 3 to4 rats per group. The arrows show the time of ACTH injection. *P<0.01; **P<0.005 compared to time 0. Figure modified from data in Spiga et al, 2011a
) RNA levels for StAR [C], cytochrome P450 side chain cleavage (CYP11A) [D], melanocortin type 2 receptor (MC2R) [E] and melanocortin receptor associated protein (MRAP) [F]. Rats were killed by decapitation at the time points indicated. Plasma ACTH levels tended to increase at 5 min. This small and non-statistically significant increase in plasma ACTH injection caused significant increases in plasma corticosterone levels, accompanied by significant increases in StAR, CYP 11A and MRAP hnRNAs, and StAR and MRAP mRNAs. No changes in MC2R mRNA were observed following the ACTH injection. Data points represent the mean and SE of the values in 3 to4 rats per group. The arrows show the time of ACTH injection. *P<0.01; **P<0.005 compared to time 0. Figure modified from data in Spiga et al, 2011a
The hnRNA responses of StAR, CYP 11A and MRAP to such small elevations in circulating ACTH indicate that transcriptional responses of the adrenal fasciculata are highly sensitive to ACTH. Moreover, there is evidence that episodes of transcription in the adrenal fasciculata can be maintained following repeated small pulses of ACTH (Spiga et al., 2011b). In the latter study, measurement of StAR, CYP11A and MRAP hnRNAs in the adrenal fasciculata 24 h after pulsatile ACTH infusion (4ng every hour), revealed levels significantly higher during the peak of the infusion compared with the descending phase of the pulse infusion. The repeated transcriptional episodes associated with each pulse of steroid secretion supports the view that these proteins undergo rapid turnover and that activation of transcription and de novo protein synthesis are part of the mechanism of pulsatility. It should be noted that the increases in plasma corticosterone, following either ACTH injection or stress, always preceded the increases in StAR, CYP 11A and MRAP hnRNA. This supports the view that rapid glucocorticoid biosynthesis and secretion depends on post-transcriptional and post-translational events, such as phosphorylation of pre-existing steroidogenic proteins (Chaudhary and Stocco, 1991; Mikami and Strott, 1986; Miller and Strauss, 1999; Pon et al., 1986). On the other hand, rapid transcriptional responses would restore mRNA pools to allow synthesis of steroidogenic proteins required for the next ultradian pulse or stress mediated secretory episode.
2. Potential mechanisms for transcriptional pulsatility
The mechanisms of episodic transcription of StAR, CYP 11A and MRAP are likely to involve both episodic patterns of ACTH secretion, as well as intrinsic molecular mechanisms at the adrenal level allowing rapid initiation and termination of the transcriptional response.
2.1 Episodic ACTH secretion
Studies in humans (Gallagher et al., 1973; Keenan et al., 2004; Weitzman et al., 1971) and in larger mammals such as horse (Cudd et al., 1995; Keenan et al., 2009) and sheep (Engler et al., 1989) have shown that plasma ACTH levels in basal conditions vary in a pulsatile manner. Similarly, the increases in circulating ACTH during stress are transient (Sarabdjitsingh et al., 2010). Thus, it is likely that the major determinant of adrenal pulsatility is the pulsatile pattern of ACTH secretion. The use of intronic qRT-PCR has made it possible to measure rapid changes in transcription in relation to the increases in circulating ACTH. The time relationship between the increases in plasma ACTH just preceding the increases in steroidogenic proteins hnRNA is consistent with the role of ACTH mediating the initiation of transcription. In keeping with the ACTH dependence of the transcriptional response, the decline in hnRNA in general coincided with the decline in plasma ACTH (Figs 1 to 3). Moreover, studies in methylprednisolone-suppressed rats have shown that pulsatile but not continuous ACTH infusion restored pulsatile corticosterone secretion and changes in hnRNA for StAR, CYP11A and MRAP (Spiga et al., 2011b).
Although the weight of the evidence indicates that pulsatile ACTH is a major determinant of adrenal pulsatility, in vitro studies in collagenase dispersed adrenal fasciculata cells or Y1 cells show that ACTH induced hnRNA for StAR or MRAP (Y Liu, L Smith, M Olah and G Aguilera, unpublished observations) start to decline in spite of sustained ACTH levels in the incubation medium. This raises the possibility that intracellular feedback mechanisms may contribute to limiting transcriptional responses.
2.2 Intracellular feedback mechanisms on steroidogenic protein transcription
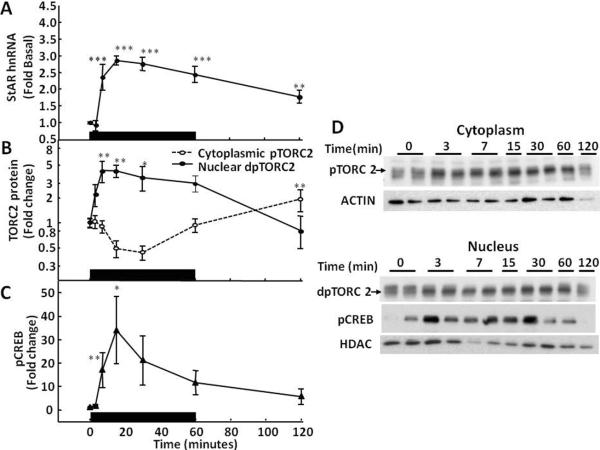
The MC2R signals through cyclic AMP-protein kinase A (PKA)-dependent pathways (Mountjoy et al., 1992). PKA rapidly induces steroid biosynthesis and secretion by activating pre-existing steroidogenic proteins, including StAR, through phosphorylation (Pon et al., 1986). A number of transcription factors, including cyclic AMP response element binding protein (CREB), cyclic AMP response element modulator (CREM), steridogenesis factor1 (SF1), CCAAT/enhancer binding protein (C/EBP) are involved in the transcriptional regulation of StAR (Christenson et al., 1999; Manna et al., 2002). PKA activates the cyclic AMP responsive element binding protein (CREB) through phosphorylation, which is a major transcription factor in promoting transcription of StAR and probably other steroidogenic proteins. Consistent with the recognized role of CREB in the transcriptional control of steroidogenic proteins, restraint stress, or ACTH injection resulted in rapid increases in phospho-CREB in nuclear proteins of the adrenal fasciculata (Figs 4-, 5- and 6-C, ). Transcriptional regulation in other systems, including regulation of corticotropin releasing hormone, inducible cyclic AMP early repressor (ICER) contributes to the self-limitation of cyclic AMP/PKA/CREB dependent transcription (Liu et al., 2006; Shepard et al., 2005). This repressor isoform of the cAMP response element modulator (CREM) (Foulkes et al., 1991; Molina et al., 1993) contains the DNA binding domain but not the transactivation domain of CREM and acts as a competitive inhibitor of pCREB-dependent transcription (Liu et al., 2006; Molina et al., 1993). ICER is expressed in the adrenal cortex and it has been shown that its expression increases in response to surgical stress in the rat adrenal gland, and that this effect is blocked by hypophysectomy and is reproduced by ACTH injection (Della Fazia et al., 1998). The generation of ICER during ACTH-stimulated steroidogenesis suggests that the repressor has a role in the regulation of transcription in the adrenal cortex, but its role in the generation of episodic transcription of StAR, Cyp 11A and MRAP will be the subject of future studies. Also, there is evidence that inactivation of the CREB co-activator, transducer of regulated CREB activity (TORC), by salt inducible kinase (SIK) may serve as an intracellular limiting mechanism for CRH transcription (Liu et al., 2010; Liu et al., 2012). It is possible that these mechanisms involved in the hypothalamic regulation of the HPA axis, also participate in transcriptional pulse generation in the adrenal cortex.
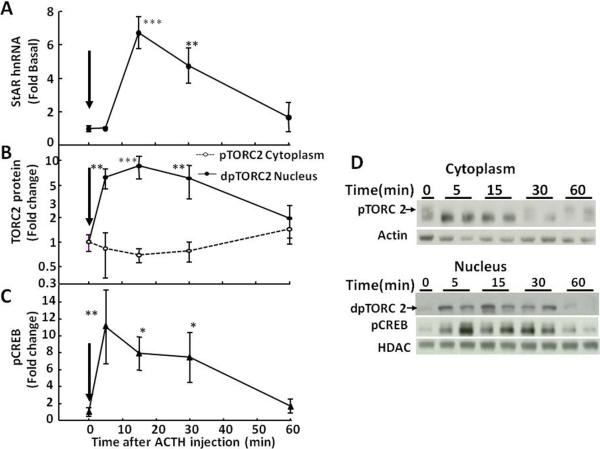
Fig 4.
Time-relationship between levels of StAR hnRNA (A), cytoplasmatic phospho-TORC2 (pTORC2,  ) and nuclear dephospho TORC2 (dpTORC2,
) and nuclear dephospho TORC2 (dpTORC2,  ) protein (B), and nuclear phospho-CREB (pCREB) (C), in the adrenal zona fasciculata of rats subjected to restraint stress Rats were subjected to restraint stress for 1h (indicated by the black bar) and killed by decapitation at the time points indicated. Significant increases in nuclear TORC2 preceded the increases in StAR hnRNA. Significant increases in nuclear phospho-CREB levels paralleled to increases in StAR hnRNA. Data points represent the mean and SE of the values in 4 rats per group. *, p<0.05; **, p<0.01; ***, p<0.001. D shows representative western blots for cytoplasmic pTORC2, nuclear dpTORC2 and pCREB. Beta actin and histone deacetylase (HDAC) were used as controls for cytoplasm and nuclear proteins, respectively.
) protein (B), and nuclear phospho-CREB (pCREB) (C), in the adrenal zona fasciculata of rats subjected to restraint stress Rats were subjected to restraint stress for 1h (indicated by the black bar) and killed by decapitation at the time points indicated. Significant increases in nuclear TORC2 preceded the increases in StAR hnRNA. Significant increases in nuclear phospho-CREB levels paralleled to increases in StAR hnRNA. Data points represent the mean and SE of the values in 4 rats per group. *, p<0.05; **, p<0.01; ***, p<0.001. D shows representative western blots for cytoplasmic pTORC2, nuclear dpTORC2 and pCREB. Beta actin and histone deacetylase (HDAC) were used as controls for cytoplasm and nuclear proteins, respectively.
Fig 5.
Time-relationship between levels of StAR hnRNA (A), cytoplasmatic phospho-TORC2 (pTORC2,  ) and nuclear dephospho TORC2 (dpTORC2,
) and nuclear dephospho TORC2 (dpTORC2,  ) protein (B), and nuclear phospho-CREB (pCREB) (C), in the adrenal zona fasciculata of rats subjected to ip injection of 5μg ACTH Rats were killed by decapitation at the time points indicated. Significant increases in nuclear dpTORC2 and pCREB preceded the increases in StAR hnRNA. Data points represent the mean and SE of the values in 4 rats per group; the arrows show the time of ACTH injection. *p<0.05; **p<0.01; ***p<0.001. D shows representative western blots for cytoplasmic pTORC2, nuclear dpTORC2 and pCREB. Beta actin and histone deacetylase (HDAC) were used as controls for cytoplasm and nuclear proteins, respectively.
) protein (B), and nuclear phospho-CREB (pCREB) (C), in the adrenal zona fasciculata of rats subjected to ip injection of 5μg ACTH Rats were killed by decapitation at the time points indicated. Significant increases in nuclear dpTORC2 and pCREB preceded the increases in StAR hnRNA. Data points represent the mean and SE of the values in 4 rats per group; the arrows show the time of ACTH injection. *p<0.05; **p<0.01; ***p<0.001. D shows representative western blots for cytoplasmic pTORC2, nuclear dpTORC2 and pCREB. Beta actin and histone deacetylase (HDAC) were used as controls for cytoplasm and nuclear proteins, respectively.
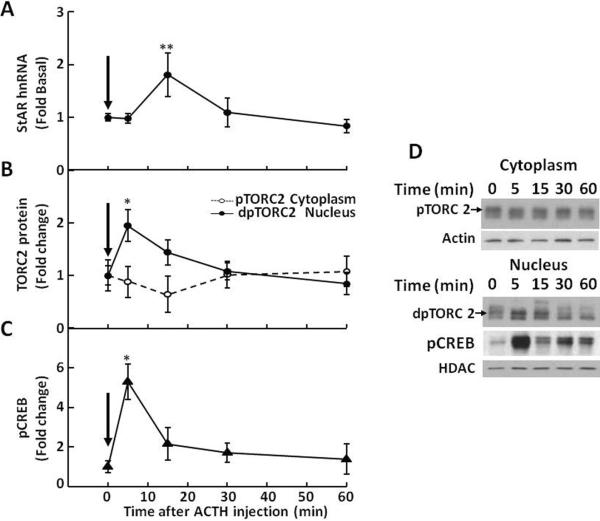
Fig 6.
Time-relationship between levels of StAR hnRNA (A), cytoplasmatic phospho-TORC2 (pTORC2,  ) and nuclear dephospho TORC2 (dpTORC2,
) and nuclear dephospho TORC2 (dpTORC2,  ) protein (B), and nuclear phospho-CREB (pCREB) (C), in the adrenal zona fasciculata of rats subjected to iv injection of 4ng ACTH Rats were killed by decapitation at the time points indicated. Significant increases in nuclear dpTORC2 and pCREB preceded the increases in StAR hnRNA. Data points represent the mean and SE of the values in 3 to 4 rats per time point. The arrows show the time of ACTH injection. *p<0.05; **p<0.01. D shows representative western blots for cytoplasmic pTORC2, nuclear dpTORC2 and pCREB. Beta actin and histone deacetylase (HDAC) were used as controls for cytoplasm and nuclear proteins, respectively. Figure modified from data in Spiga et al, 2011a
) protein (B), and nuclear phospho-CREB (pCREB) (C), in the adrenal zona fasciculata of rats subjected to iv injection of 4ng ACTH Rats were killed by decapitation at the time points indicated. Significant increases in nuclear dpTORC2 and pCREB preceded the increases in StAR hnRNA. Data points represent the mean and SE of the values in 3 to 4 rats per time point. The arrows show the time of ACTH injection. *p<0.05; **p<0.01. D shows representative western blots for cytoplasmic pTORC2, nuclear dpTORC2 and pCREB. Beta actin and histone deacetylase (HDAC) were used as controls for cytoplasm and nuclear proteins, respectively. Figure modified from data in Spiga et al, 2011a
2.2.1 Transducer of regulated CREB activity (TORC)
For a number of CREB regulated genes, CREB alone is not sufficient to activate transcription but it requires the CREB co-activator, transducer of CREB regulated activity (TORC), also called CREB regulated transcription co-activator (CRTC) (Conkright et al, 2003; Iourgenko et al, 2003). Three isoforms of TORC have been identified, TORC 1, 2 and 3. In basal conditions, TORC is in the cytoplasm bound to the scaffolding protein, 14-3-3, in a phosphorylated state by the TORC kinase, salt inducible kinase (SIK), which has two isoforms, SIK1 and SIK2. PKA-dependent phosphorylation of SIK results in its inactivation, thus allowing dephosphorylation of TORC and its translocation to the nucleus, where it regulates CREB- dependent transcription. The TORC/SIK system has been implicated in adrenal regulation (Takemori and Okamoto, 2008). SIK is present in the adrenal and studies in cell lines have shown that its inhibition by the protein kinase inhibitor, staurosporine, induces StAR and steroidogenic enzymes parallel to TORC dephosphorylation (Takemori et al., 2007). To determine whether the TORC/SIK system contributes to transcriptional pulse generation in the adrenal, it was important to first examine the effects of ACTH in TORC activation in relationship with the initiation and termination of the transcriptional response of steroidogenic proteins. TORC dephosphorylation following PKA inactivation of SIK can be detected in the western blot by a slightly more rapid migration in the gel electrophoresis (Conkright et al, 2003).
Western blot analysis of cytoplasmic and nuclear proteins from adrenal fasciculata in rats subjected to restraint stress using TORC2 showed a reduction in the upper band corresponding to phosphorylated TORC and an increase in a lower band corresponding to dephospho-TORC at the first time point measured, 3 min (Fig-4-B and D). In contrast, basal nuclear dephospho-TORC2 levels were low, started to increase by 3 min, and reached a maximum by 7.5 min, concomitant with the rise in StAR hnRNA (Fig 4-B and D). Both nuclear TORC2 levels and StAR hnRNA had declined to basal levels by 120 min. A similar temporal pattern with increases in nuclear TORC 2 preceding the increases in StAR hnRNA were observed following ACTH injection (Figs 5 and 6). A single injection of ACTH 1–24, 5μ-g, i.p., in methylprednisolone-suppressed rats at the time points indicated in the figures show rapid decreases in cytoplasmic phospho-TORC2 parallel to increases in nuclear dephosphorylated TORC2. As seen in Fig 5, significant increases in nuclear TORC2 were already evident at 5 min (B and D), while the increases in StAR hnRNA became evident at the next time point, 15min (A). As previously shown in Fig 2, hnRNA levels for CYP11A and MRAP also raised after TORC2 had migrated to the nucleus. Smaller changes with an identical pattern were found with the ACTH injection mimicking ultradian variations, 4ng iv (Figure 6) (Spiga et al., 2011b). The temporal pattern of TORC2 translocation to the nucleus also precedes the induction of CYP11A and MRAP hnRNA shown in Figs 1 to 3, suggesting that TORC is involved in the transcriptional regulation of all of these proteins essential for steroidogenesis.
Prior to the discovery of the CREB co-activator TORC, studies using reporter gene assays showed that SIK overexpression down-regulated the expression of several steroidogenic proteins including StAR, CYP11A, CYP11B1 and CYP11B2 (Lin et al., 2001). Later it became clear that SIK phosphorylates TORC and that SIK over-expression phosphorylated TORC in adrenal cell lines (Katoh et al., 2004). Figure 7 shows that both SIK 1 and SIK2 are present in the adrenal zone fasciculata in rats. Consistent with other systems such as the hypothalamic paraventricular nucleus and the pineal gland, SIK 1 is highly inducible (Kanyo et al., 2009; Liu et al., 2012). Injection of ACTH, 5 μg, in methylprednisolone-suppressed rats caused marked increases in SIK mRNA of about 10-fold by 15 min and 30-fold by 30 min which returned to basal levels by 1 h (Fig 7). The lower ACTH injection dose mimicking an ultradian pulse (4ng) was also shown to increase SIK1 mRNA at 30 min (Spiga et al., 2011a). In contrast SIK2 showed a slight but significant increase (less than 2-fold) after 30 and 60 min (Fig 7).
Fig 7.
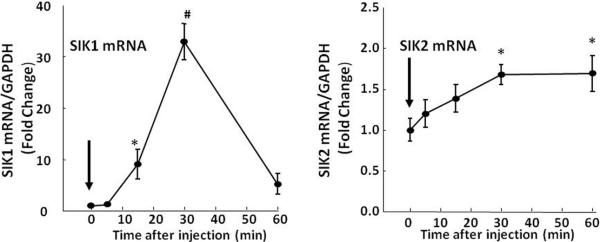
Time course of the effect of ACTH, 5μg, i.p., on levels of SIK1 and SIK2 mRNA in the adrenal zona fasciculata SIK1 mRNA was highly inducible increasing by about 30-fold by 30 min, while SIK2 increased by less than 2-fold. *,p<0.05; **, p< 0.01; ***, p<0.001. Data points represent the mean and SE of the values in 4 rat per group.
Although there is unequivocal evidence that induction of steroidogenic proteins transcription by stress of ACTH is associated with TORC trafficking to the nucleus, the precise role of TORC in the regulation of these proteins expression is still under investigation. The temporal sequence of nuclear translocation of TORC preceding hnRNA induction episodes supports a role for the co-activator in transcriptional pulse generation. However, elucidation of the effects of TORC/SIK overexpression and knock down, as well as the ability of the promoters to recruit TORC will be critical to elucidate this question.
Conclusions
Increasing evidence indicates that episodic patterns of glucocorticoid secretion are essential for maintaining transcriptional responsiveness of glucocorticoid-dependent genes (Sarabdjitsingh et al., 2010; Spiga et al., 2011b). The recent introduction of intronic qRT-PCR to measure primary transcript levels in adrenal tissue has made it possible to detect rapid changes in transcription of key proteins involved in steroidogenesis (Liu et al., 2008; Spiga et al., 2011a; Spiga et al., 2011b). The studies described above clearly demonstrate that the transient increases in glucocorticoid secretion induced by stress are associated with transcriptional episodes of steroidogenic proteins including StAR, CYP11A and MRAP. The ability of ACTH injections providing plasma levels in the stress range to mimic ultradian pulses or stress-induced transcriptional episodes suggests that episodic ACTH secretion is largely responsible for the transcriptional events. The repeated transcriptional episodes associated with each pulse of steroid secretion suggest rapid turnover of critical steroidogenic proteins and that activation of transcription and de novo protein synthesis are part of the mechanism of pulsatility.
In addition to pulsatile ACTH secretion it is likely that intrinsic adrenal mechanisms involving intracellular feedback events contribute to episodic transcription. A potential mechanism includes ACTH-mediated induction of inducible cyclic AMP early repressor (ICER). The demonstration that transcriptional episodes are preceded by rapid translocation of the CREB co-activator, transducer of regulated CREB activity (TORC) to the nucleus, suggests that ACTH mediated activation of TORC is necessary for transcriptional initiation. Similarly the decline of TORC in the nucleus in conjunction with the induction of the TORC kinase, SIK1, implies that TORC inactivation could contribute to termination of the transcriptional response. Current evidence indicates that in addition to ACTH pulsatility, intra-adrenal mechanisms, such as ICER and the TORC/SIK system, could act as on/off switches for rapid activation and inactivation of steroidogenic protein transcription, including StAR. However, further studies are clearly needed to elucidate the relative role of these mechanisms on the physiological control of transcriptional episodes in the adrenal.
Highlights
The transient increases in glucocorticoid secretion induced by stress are associated with episodes of transcriptional activity of proteins necessary for steroidogenesis
Episodic transcription of steroidogenic proteins can be reproduced by ACTH injections providing pulses of circulating levels either in the stress or basal ultradian levels
ACTH causes rapid translocation of the cyclic AMP responsive element binding protein (CREB) co-activator, transducer of regulated CREB activity, TORC, to the nucleus, preceding the initiation of each steroidogenic protein transcriptional episode.
The time correlation with sequential TORC translocation to the nucleus, episodes of steroidogenic protein transcription, and induction of the TORC kinase, salt inducible kinase (SIK) suggest that activation and inactivation of TORC may contribute to the generation of transcriptional pulses.
Highlights.
Basal and stress-induced adrenal glucocorticoid secretion are episodic
Episodic secretion parallels episodes of steroidogenic protein gene transcription
ACTH mediates ultradian and stress-induced transcriptional episodes
Nuclear localization of the co-activator, TORC, precedes transcriptional activation
TORC regulation may play a key role in transcriptional pulse generation
Abbreviations
- ACTH
adrenocorticotropic hormone
- CRH
corticotrophin releasing hormone
- StAR
steroidogenic acute regulatory protein
- CYP11A
cytochrome P450 11A or side chain cleavage enzyme
- CYP11B1
cytochrome P450 11beta 1
- CYP11B2
cytochrome P450 11beta 2
- CREB
cyclic AMP response element binding protein
- CREM
cyclic AMP response element modulator
- hn
heteronuclear RNA
- ICER
inducible cyclic AMP early repressor
- HPA axis
hypothalamic pituitary adrenal axis
- MC2R
melanocortin type 2 receptor or ACTH receptor
- MRAP
melanocortin receptor associated protein
- PKA
protein kinase A
- qRT-PCR
real time polymerase chain reaction
- RNA
ribonucleic acid
- SF1
steroidogenic factor 1
- SIK
salt inducible kinase
- TORC
transducer of regulated CREB activity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera G, Volpi S, Rabadan-Diehl C. Transcriptional and post-transcriptional mechanisms regulating the rat pituitary vasopressin V1b receptor gene. J Mol Endocrinol. 2003;30:99–108. doi: 10.1677/jme.0.0300099. [DOI] [PubMed] [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank. Nucleic Acids Res. 2005;33:D34–38. doi: 10.1093/nar/gki063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush IE. Species differences in adrenocortical secretion. Journal of Endocrinology. 1953;9:95–NP. doi: 10.1677/joe.0.0090095. [DOI] [PubMed] [Google Scholar]
- Carnes M, Lent S, Feyzi J, Hazel D. Plasma adrenocorticotropic hormone in the rat demonstrates three different rhythms within 24 h. Neuroendocrinology. 1989;50:17–25. doi: 10.1159/000125197. [DOI] [PubMed] [Google Scholar]
- Chaudhary LR, Stocco DM. Effect of different steroidogenic stimuli on protein phosphorylation and steroidogenesis in MA-10 mouse Leydig tumor cells. Biochim Biophys Acta. 1991;1094:175–184. doi: 10.1016/0167-4889(91)90006-j. [DOI] [PubMed] [Google Scholar]
- Christenson LK, Johnson PF, McAllister JM, Strauss JF., 3rd CCAAT/enhancer-binding proteins regulate expression of the human steroidogenic acute regulatory protein (StAR) gene. J Biol Chem. 1999;274:26591–26598. doi: 10.1074/jbc.274.37.26591. [DOI] [PubMed] [Google Scholar]
- Churchill PF, Kimura T. Topological studies of cytochromes P-450scc and P-45011 beta in bovine adrenocortical inner mitochondrial membranes. Effects of controlled tryptic digestion. J Biol Chem. 1979;254:10443–10448. [PubMed] [Google Scholar]
- Cone RD, Mountjoy KG, Robbins LS, Nadeau JH, Johnson KR, Roselli-Rehfuss L, Mortrud MT. Cloning and functional characterization of a family of receptors for the melanotropic peptides. Ann N Y Acad Sci. 1993;680:342–363. doi: 10.1111/j.1749-6632.1993.tb19694.x. [DOI] [PubMed] [Google Scholar]
- Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, Montminy M. TORCs: transducers of regulated CREB activity. Mol Cell. 2003;12:413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Cudd TA, LeBlanc M, Silver M, Norman W, Madison J, Keller-Wood M, Wood CE. Ontogeny and ultradian rhythms of adrenocorticotropin and cortisol in the late-gestation fetal horse. J Endocrinol. 1995;144:271–283. doi: 10.1677/joe.0.1440271. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Levin N, Walker CD, Bradbury MJ, Suemaru S, Scribner KS. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann N Y Acad Sci. 1994;746:22–31. doi: 10.1111/j.1749-6632.1994.tb39206.x. [DOI] [PubMed] [Google Scholar]
- Della Fazia MA, Servillo G, Foulkes NS, Sassone-Corsi P. Stress-induced expression of transcriptional repressor ICER in the adrenal gland. FEBS Letters. 1998;434:33–36. doi: 10.1016/s0014-5793(98)00944-2. [DOI] [PubMed] [Google Scholar]
- Desir D, van Cauter E, Golstein J, Fang VS, Leclercq R, Refetoff S, Copinschi G. Circadian and ultradian variations of ACTH and cortisol secretion. Horm Res. 1980;13:302–316. doi: 10.1159/000179297. [DOI] [PubMed] [Google Scholar]
- Engler D, Pham T, Fullerton MJ, Clarke IJ, Funder JW. Evidence for an ultradian secretion of adrenocorticotropin, beta-endorphin and alpha-melanocyte-stimulating hormone by the ovine anterior and intermediate pituitary. Neuroendocrinology. 1989;49:349–360. doi: 10.1159/000125139. [DOI] [PubMed] [Google Scholar]
- Ferguson JJ., Jr. Protein Synthesis and Adrenocorticotropin Responsiveness. J Biol Chem. 1963;238:2754–2759. [PubMed] [Google Scholar]
- Foulkes NS, Borrelli E, Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991;64:739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Lundblad JR, Pritchett DB, Wilcox JN, Roberts JL. Regulation of proopiomelanocortin gene transcription in individual cell nuclei. Science. 1986;234:1265–1269. doi: 10.1126/science.3775385. [DOI] [PubMed] [Google Scholar]
- Gallagher TF, Yoshida K, Roffwarg HD, Fukushima DK, Weitzman ED, Hellman L. ACTH and cortisol secretory patterns in man. J Clin Endocrinol Metab. 1973;36:1058–1068. doi: 10.1210/jcem-36-6-1058. [DOI] [PubMed] [Google Scholar]
- Garren LD, Ney RL, Davis WW. Studies on the role of protein synthesis in the regulation of corticosterone production by adrenocorticotropic hormone in vivo. Proc Natl Acad Sci U S A. 1965;53:1443–1450. doi: 10.1073/pnas.53.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hum DW, Miller WL. Transcriptional regulation of human genes for steroidogenic enzymes. Clin Chem. 1993;39:333–340. [PubMed] [Google Scholar]
- Ivell R, Tillmann G, Wang H, Nicol M, Stewart PM, Bartlick B, Walther N, Mason JI, Morley SD. Acute regulation of the bovine gene for the steroidogenic acute regulatory protein in ovarian theca and adrenocortical cells. J Mol Endocrinol. 2000;24:109–118. doi: 10.1677/jme.0.0240109. [DOI] [PubMed] [Google Scholar]
- Iourgenko V, Zhang W, Mickanin C, Daly I, Jiang C, Hexham JM, Orth AP, Miraglia L, Meltzer J, Garza D, Chirn GW, McWhinnie E, Cohen D, Skelton J, Terry R, Yu Y, Bodian D, Buxton FP, Zhu J, Song C, Labow MA. Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc Natl Acad Sci USA. 2003;100:12147–12152. doi: 10.1073/pnas.1932773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyo R, Price DM, Chik CL, Ho AK. Salt-inducible kinase 1 in the rat pinealocyte: adrenergic regulation and role in arylalkylamine N-acetyltransferase gene transcription. Endocrinology. 2009;150:4221–4230. doi: 10.1210/en.2009-0275. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Takemori H, Min L, Muraoka M, Doi J, Horike N, Okamoto M. Salt-inducible kinase-1 represses cAMP response element-binding protein activity both in the nucleus and in the cytoplasm. Eur J Biochem. 2004;271:4307–4319. doi: 10.1111/j.1432-1033.2004.04372.x. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Roelfsema F, Veldhuis JD. Endogenous ACTH concentration-dependent drive of pulsatile cortisol secretion in the human. Am J Physiol Endocrinol Metab. 2004;287:E652–661. doi: 10.1152/ajpendo.00167.2004. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Alexander S, Irvine C, Veldhuis JD. Quantifying nonlinear interactions within the hypothalamo-pituitary-adrenal axis in the conscious horse. Endocrinology. 2009;150:1941–1951. doi: 10.1210/en.2008-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoux JG, Fleury A, Ducharme L. The acute and chronic effects of adrenocorticotropin on the levels of messenger ribonucleic acid and protein of steroidogenic enzymes in rat adrenal in vivo. Endocrinology. 1998;139:3913–3922. doi: 10.1210/endo.139.9.6196. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. European Journal of Pharmacology. 2008;583:255–262. doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- Lin X, Takemori H, Katoh Y, Doi J, Horike N, Makino A, Nonaka Y, Okamoto M. Salt-inducible kinase is involved in the ACTH/cAMP-dependent protein kinase signaling in Y1 mouse adrenocortical tumor cells. Mol Endocrinol. 2001;15:1264–1276. doi: 10.1210/mend.15.8.0675. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kalintchenko N, Sassone-Corsi P, Aguilera G. Inhibition of corticotrophin-releasing hormone transcription by inducible cAMP-early repressor in the hypothalamic cell line, 4B. J Neuroendocrinol. 2006;18:42–49. doi: 10.1111/j.1365-2826.2005.01383.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kamitakahara A, Kim AJ, Aguilera G. Cyclic adenosine 3',5'-monophosphate responsive element binding protein phosphorylation is required but not sufficient for activation of corticotropin-releasing hormone transcription. Endocrinology. 2008;149:3512–3520. doi: 10.1210/en.2008-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Coello AG, Grinevich V, Aguilera G. Involvement of transducer of regulated cAMP response element-binding protein activity on corticotropin releasing hormone transcription. Endocrinology. 2010;151:1109–1118. doi: 10.1210/en.2009-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Poon V, Sanchez-Watts G, Watts AG, Takemori H, Aguilera G. Salt-inducible kinase is involved in the regulation of corticotropin-releasing hormone transcription in hypothalamic neurons in rats. Endocrinology. 2012;153:223–233. doi: 10.1210/en.2011-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol. 2002;16:184–199. doi: 10.1210/mend.16.1.0759. [DOI] [PubMed] [Google Scholar]
- Manna PR, Stocco DM. Crosstalk of CREB and Fos/Jun on a single cis-element: transcriptional repression of the steroidogenic acute regulatory protein gene. J Mol Endocrinol. 2007;39:261–277. doi: 10.1677/JME-07-0065. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Metherell LA, Chapple JP, Cooray S, David A, Becker C, Ruschendorf F, Naville D, Begeot M, Khoo B, Nurnberg P, Huebner A, Cheetham ME, Clark AJ. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37:166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- Mikami K, Strott CA. Cyclic AMP-dependent protein kinase activity and protein phosphorylation in zones of the adrenal cortex. Biochem Biophys Res Commun. 1986;138:895–901. doi: 10.1016/s0006-291x(86)80580-0. [DOI] [PubMed] [Google Scholar]
- Miller WL, Strauss JF., 3rd Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol. 1999;69:131–141. doi: 10.1016/s0960-0760(98)00153-8. [DOI] [PubMed] [Google Scholar]
- Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- Mountjoy K, Robbins L, Mortrud M, Cone R. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Munck A, Naray-Fejes-Toth A. Glucocorticoids and stress: permissive and suppressive actions. Ann N Y Acad Sci. 1994;746:115–130. doi: 10.1111/j.1749-6632.1994.tb39221.x. [DOI] [PubMed] [Google Scholar]
- Pon LA, Hartigan JA, Orme-Johnson NR. Acute ACTH regulation of adrenal corticosteroid biosynthesis. Rapid accumulation of a phosphoprotein. J Biol Chem. 1986;261:13309–13316. [PubMed] [Google Scholar]
- Rabadan-Diehl C, Lolait SJ, Aguilera G. Regulation of pituitary vasopressin V1b receptor mRNA during stress in the rat. J Neuroendocrinol. 1995;7:903–910. doi: 10.1111/j.1365-2826.1995.tb00734.x. [DOI] [PubMed] [Google Scholar]
- Rabadan-Diehl C, Kiss A, Camacho C, Aguilera G. Regulation of messenger ribonucleic acid for corticotropin releasing hormone receptor in the pituitary during stress. Endocrinology. 1996;137:3808–3814. doi: 10.1210/endo.137.9.8756551. [DOI] [PubMed] [Google Scholar]
- Roy S, Roy SJ, Pinard S, Taillefer LD, Rached M, Parent JL, Gallo-Payet N. Mechanisms of melanocortin-2 receptor (MC2R) internalization and recycling in human embryonic kidney (hek) cells: identification of Key Ser/Thr (S/T) amino acids. Mol Endocrinol. 2011;25:1961–1977. doi: 10.1210/me.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabdjitsingh RA, Conway-Campbell BL, Leggett JD, Waite EJ, Meijer OC, de Kloet ER, Lightman SL. Stress responsiveness varies over the ultradian glucocorticoid cycle in a brain-region-specific manner. Endocrinology. 2010;151:5369–5379. doi: 10.1210/en.2010-0832. [DOI] [PubMed] [Google Scholar]
- Sewer MB, Waterman MR. ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Tech. 2003;61:300–307. doi: 10.1002/jemt.10339. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Liu Y, Sassone-Corsi P, Aguilera G. Role of glucocorticoids and cAMP-mediated repression in limiting corticotropin-releasing hormone transcription during stress. J Neurosci. 2005;25:4073–4081. doi: 10.1523/JNEUROSCI.0122-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga F, Liu Y, Aguilera G, Lightman SL. Temporal effect of adrenocorticotrophic hormone on adrenal glucocorticoid steroidogenesis: involvement of the transducer of regulated cyclic AMP-response element-binding protein activity. J Neuroendocrinol. 2011a;23:136–142. doi: 10.1111/j.1365-2826.2010.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga F, Waite EJ, Liu Y, Kershaw YM, Aguilera G, Lightman SL. ACTH-dependent ultradian rhythm of corticosterone secretion. Endocrinology. 2011b;152:1448–1457. doi: 10.1210/en.2010-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11:1093–1102. doi: 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem Pharmacol. 1996;51:197–205. doi: 10.1016/0006-2952(95)02093-4. [DOI] [PubMed] [Google Scholar]
- Takemori H, Kanematsu M, Kajimura J, Hatano O, Katoh Y, Lin XZ, Min L, Yamazaki T, Doi J, Okamoto M. Dephosphorylation of TORC initiates expression of the StAR gene. Mol Cell Endocrinol. 2007;265–266:196–204. doi: 10.1016/j.mce.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Takemori H, Okamoto M. Regulation of CREB-mediated gene expression by salt inducible kinase. J Steroid Biochem Mol Biol. 2008;108:287–291. doi: 10.1016/j.jsbmb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Mezey E, Siegel RE. Vasopressin and oxytocin mRNAs in adrenalectomized and Brattleboro rats: analysis by quantitative in situ hybridization histochemistry. Brain Res. 1986;387:231–241. doi: 10.1016/0169-328x(86)90029-x. [DOI] [PubMed] [Google Scholar]