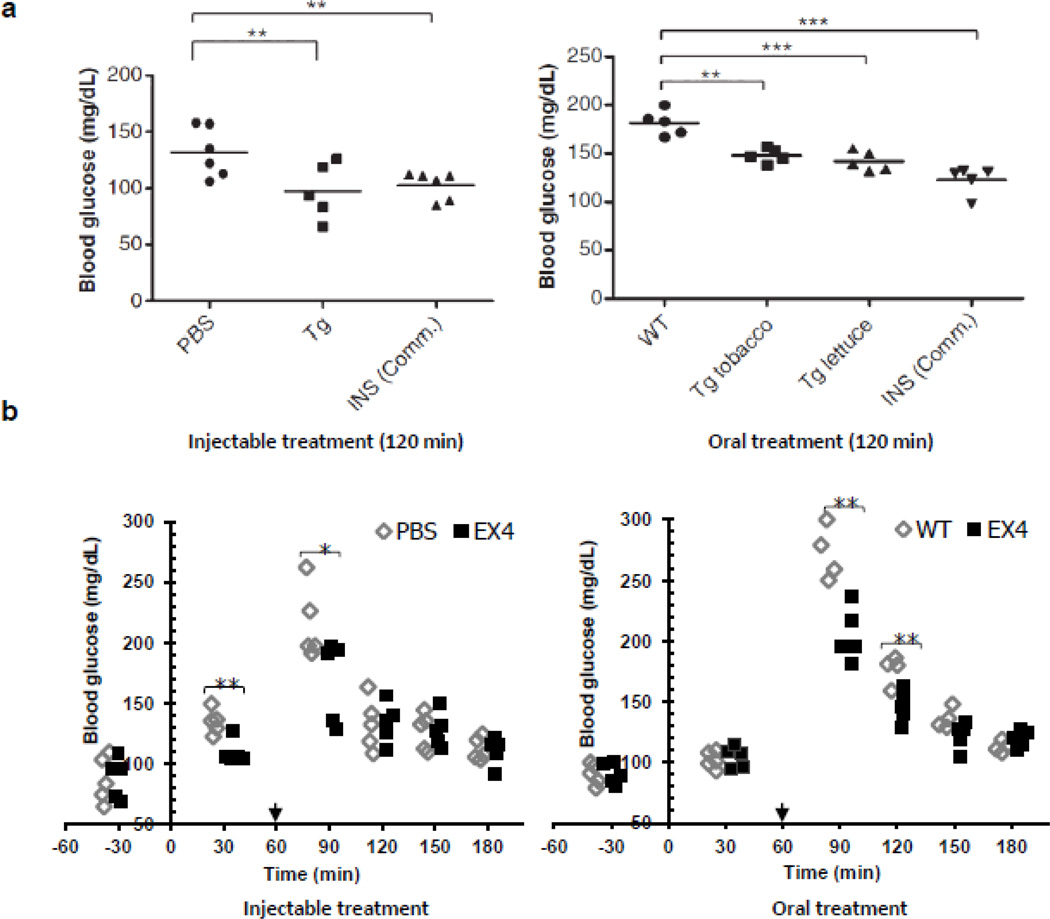
Fig. 2.
Functional evaluation of CTB-proinsulin and CTB-exendin-4 delivered by injection or oral gavage in mice. (a) Blood glucose level after intraperitoneal (IP) injection of purified CTBP-Fx3, commercial insulin (INS Comm.) and oral delivery of control (WT), plant cells expressing CTB-PFx3 (Tg tobacco, Tg lettuce). © Plant Biotechnology Journal, Society for Experimental Biology, Association of Applied Biologists and Blackwell Publishing Ltd, Plant Biotechnology Journal (2010) 9: 585–598 [20]. (b) Blood glucose level after intraperitoneal (IP) injection of purified commercial EX4 and oral administration of untransformed tobacco (WT), tobacco expressing CTB-EX4 (EX4). PBS was also injected as negative control. Arrows indicate the time point of glucose spike. *P < 0.05, **P < 0.01, ***P<0.001. © Plant Biotechnology Journal, Society for Experimental Biology, Association of Applied Biologists and Blackwell Publishing Ltd, Plant Biotechnology Journal (2012): In Press [25].