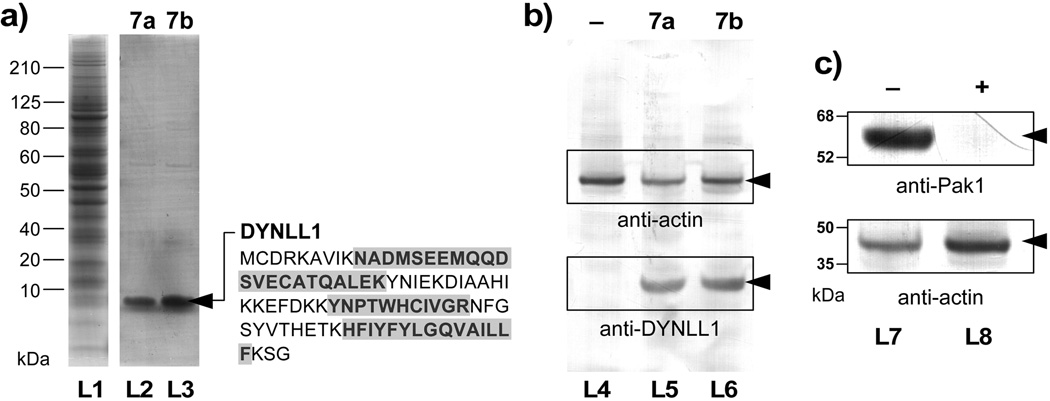
Figure 4.
Forward affinity studies identify dynein light chain type 1 (DYNLL1) as a target of aplysqualenol A (1a). (a) SDS–PAGE gels of HCT–116 cell lysate (lane L1) and the fractions that were pulled down with resins 7a (lane L2) and 7b (lane L3). A band corresponding to a <10 kDa protein was observed in the bound fractions from both resins 7a and 7b. (b) Western blot analysis using an anti–DYNLL1 mAb confirms the presence of DYNLL1 protein in the bound fractions from 7a (lane L5) and 7b (lane L6). Resins that lack 1a (lane L4) as given by a sample of resin 5 that was capped with glycine methyl ester did not contain DYNLL1. Actin was added to each fraction as loading control. (c) Affigel resin bearing 2.4±0.2 mg/mL of DYNLL1 was capable of isolating Pak1 from HCT–116 cell lysate. Western blots depict the isolation of Pak1 in DYNLL1–affinity fraction of pure lysates (–, lane L7). In contrast, the same lysate treated with 20 µM 1a lost the ability to sequester Pak1 (+, lane L7), indicating that 1a blocked the interaction between DYNLL1 and Pak1.[16]