Abstract
Angiostrongylus cantonensis is a zoonotic pathogen that occasionally causes human angiostrongyliasis; its main clinical manifestation is eosinophilic meningitis. This report defines the concept of intrathecal activation of complement as evidence of intrathecal synthesis of major immunoglobulins during this disease. Details are presented of the activation of complement system components in cerebrospinal fluid, and their application to our understanding of this tropical disease, which is emerging in the Western hemisphere. Intrathecal synthesis of at least one of the major immunoglobulins and a wide spectrum of patterns may be observed. Although intrathecal synthesis of C3c is always present, C4 intrathecal synthesis does not occur in every patient. The diversity of intrathecal synthesis and activation of the different complement pathways enables their division into three variant groups (A, B, and C). Variant group A includes the classical and/or lectin pathway and involves two or more major immunoglobulins with C3 and C4 intrathecal synthesis. Variant group B involves C4 in cerebrospinal fluid that comes from blood in the intrathecal activation of the classical pathway. Variant group C includes the alternative pathway.
Introduction
Angiostrongylus cantonensis is a zoonotic pathogen that occasionally causes human angiostrongyliasis; its main clinical manifestation is eosinophilic meningitis. The nematode worm A. cantonensis was discovered in the pulmonary arteries and hearts of domestic rats in Guangzhou, China.1
Angiostrongylus cantonensis is endemic to southern Asia,2 the Pacific islands,3 and the Caribbean islands,4 and probably in South America.5,6 The life cycle of this nematode involves rats as definitive hosts; molluscs as intermediate hosts; and crustaceans (prawns and land crabs), predacious land planarians, frogs, and monitor lizards as paratenic (transfer or transport) hosts. Humans acquire the parasite after eating intermediate or paratenic hosts, or vegetables that contain infective larvae (the third stage) of the worm.1
Cerebral angiostrongyliasis is generally diagnosed from the patient's clinical history and cerebrospinal fluid (CSF) eosinophilia, and is supported by a history of possible exposure to infective larvae. The gold standard in diagnosis, although often difficult, is detection of the disease agent. Angiostrongyliasis should be considered a human disease that is sometimes fatal. Frequently, the infection occurs in outbreaks with a variable number of cases.7–9 Human angiostrongyliasis was first reported in the Western Hemisphere in Cuba in 1981,10 and then in Haiti, Jamaica,11 Brazil,12 Dominican Republic,13 and most recently in Ecuador.14
Complement, a central component of the innate immune system, is comprised of at least 35 proteins that collaborate in an intricate manner to eliminate microorganisms and remove apoptotic cells. Complement also serves as a natural adjuvant, enhancing and directing the adaptive immune response.15 Although the final effect of activation of this system is the destruction of microorganisms, it may also lead to host tissue damage. The complement system has been regarded as being comprised of three activation pathways: 1) the classical pathway initiated by C1q, 2) the lectin pathway initiated by mannan-binding lectin (MBL), and 3) the alternative pathway, which is triggered by spontaneous C3 hydrolysis or direct pattern recognition of properdin.15,16
It was recently demonstrated that in murine experimental cerebral malaria, the classical or alternative complement pathways are not required for disease development,17 apparently because activation of C5 occurs via coagulation enzymes of the extrinsic protease pathway. This pathway should be considered a fourth complement pathway.
The use of Reibergrams (immunoglobulin CSF/serum quotient Reiber diagrams) has been reported for angiostrongyliasis and other infectious diseases.18,19 Complement component C3c is a degradation and stable product of C3; it is currently quantified instead of the whole C3 molecule because its presence indicates that C3 was biologically used in the immune response. The use of C3c and C4 Reibergrams has also been reported;20,21 these are used to confirm that local complement factor was synthesized in the central nervous system (CNS).20,21 The validity of this option is supported by the molecular flux/CSF flow theory.22,23 Recently, different major immunoglobulin patterns in patients with angiostrongyliasis patients in Ecuador and Cuba with dissimilar neuroimmunologic and epidemiologic features, have been reported.5,6 Other reports described intrathecal synthesis patterns of major immunoglobulins24 and C3 and C4 in patients with angiostrongyliasis.20,21
This report reviews the different patterns of this immune response and defines the concept of intrathecal activation of complement. It also aims to explain the relevance of these patterns during oligoclonal intrathecal synthesis, as well as the activation in CSF of one or more complement system components in meningoencephalitis caused by A. cantonensis.
Reibergrams.
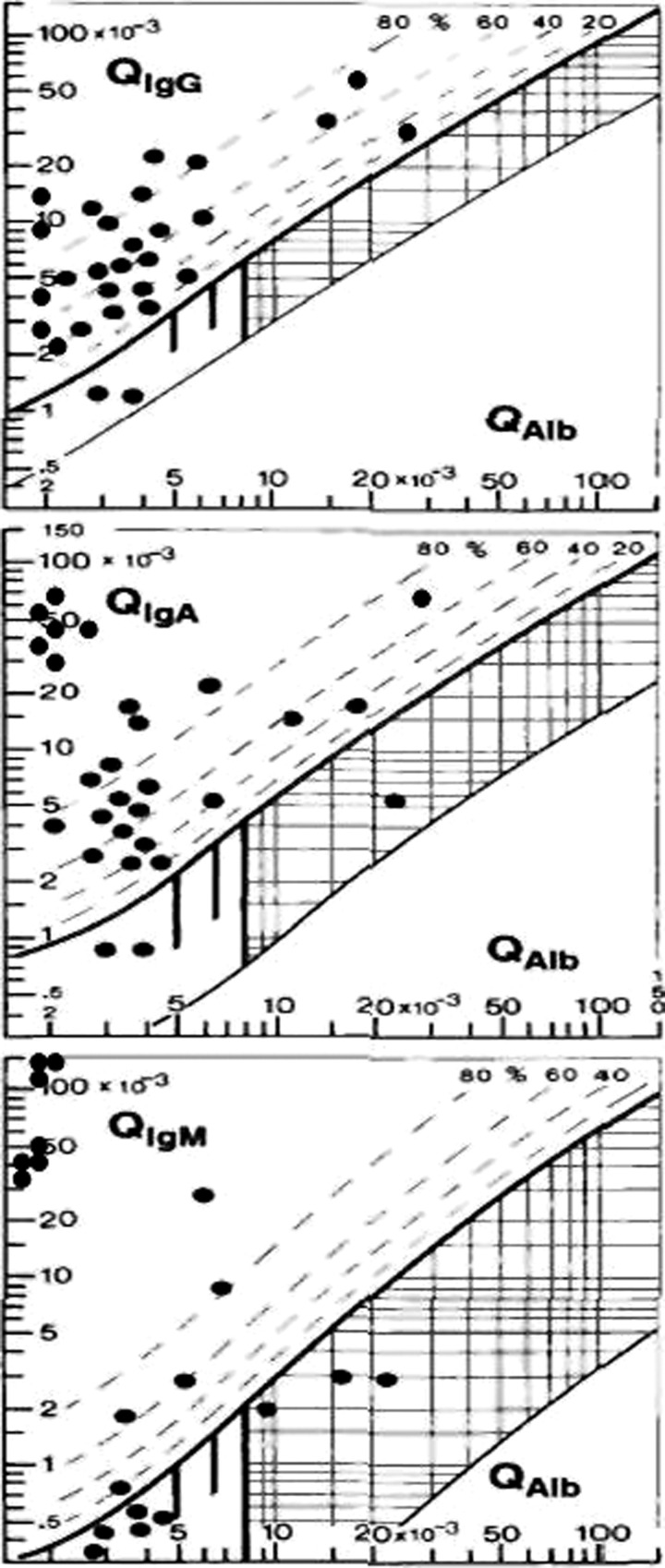
Reference to CSF/serum quotient diagrams or Reibergrams (Figure 1) enables an understanding of the intrathecal synthesis findings seen in angiostrongyliasis. The Reibergram was introduced for quantification of major immunoglobulin intrathecal synthesis,24 IgG subclasses,25 IgE,26 and some components of the complement system.20,21
Figure 1.
Reibergram of major immunoglobulins in patients with eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis.24 Copyright by the American Society for Microbiology.
This procedure is based on molecular diffusion/CSF flow theory; its fundamental principle is that a decrease in flow rate is always accompanied by an increase in molecular diffusion from blood to CSF. Reibergrams are diagrams that analyze in an integrated way the function of the blood-CSF barrier and intrathecal protein synthesis. They also aid in the diagnosis of CNS diseases associated with specific patterns of immunoglobulin response.22,23
The upper hyperbolic curve (thick line) represents the discrimination line between brain-derived and blood-derived proteins. Values above this upper line represent intrathecal specific protein synthesis such as major immunoglobulins (Figure 1), C3c (Figure 2), or C4 (Figure 3).
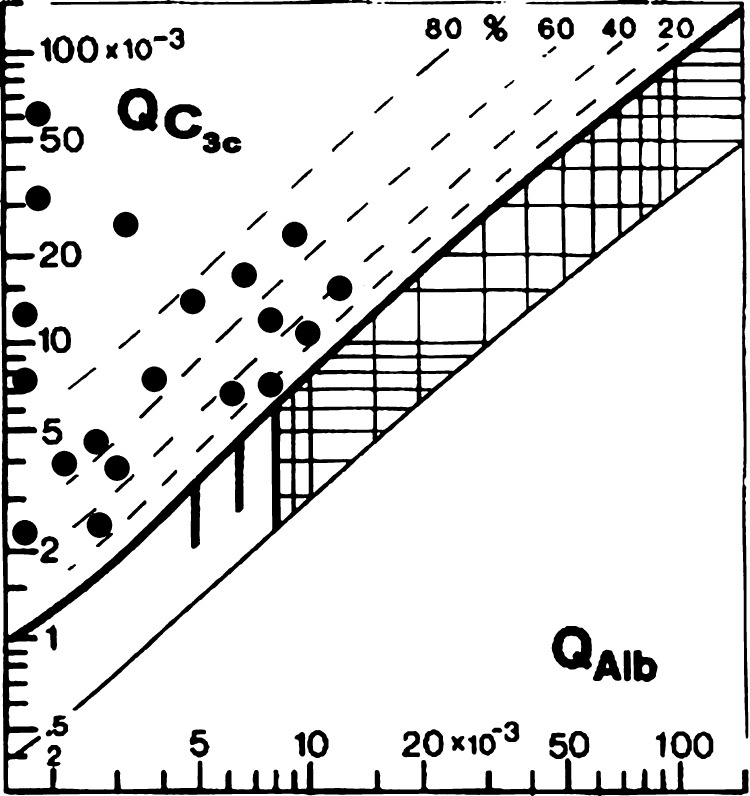
Figure 2.
Reibergram for C3c in patients with meningoencephalitis caused by Angiostrongylus cantonensis.
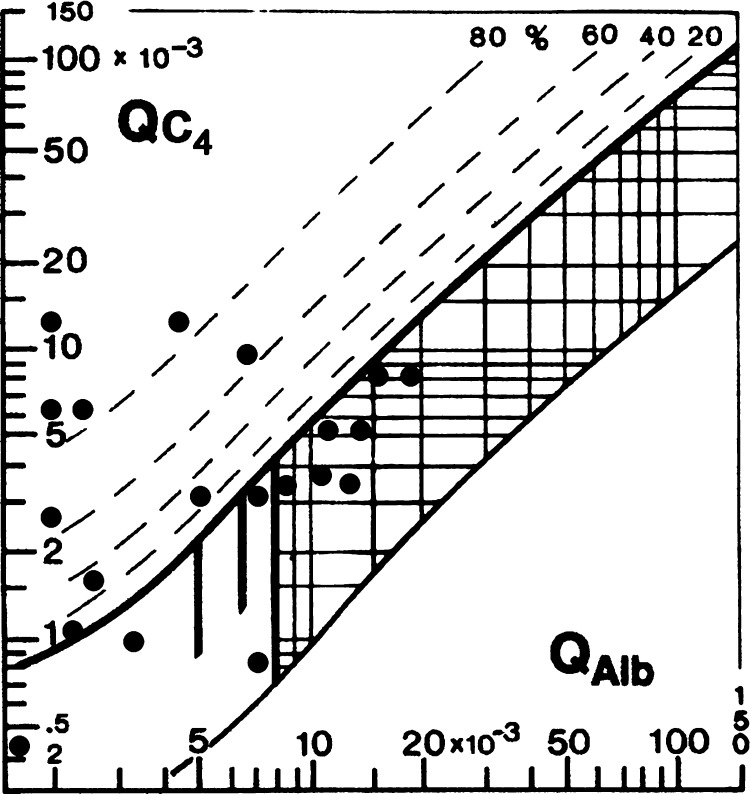
Figure 3.
Reibergam for C4 in cerebral angiostrongyliasis.
The dashed lines indicate the extent of intrathecal synthesis as intrathecal fractions (C4IF, C3cIF, or immunoglobulin IF) with 20%, 40%, 60%, and 80% of the measured total specific protein concentration in CSF, with reference to the discrimination line as 0% intrathecal synthesis. The limit of the reference range for QAlb between reference (normal) and increased CSF protein concentrations caused by blood-CSF barrier dysfunction is indicated by the age-dependent vertical lines: At QAlb = 5 × 10−3 (≤ 15 years), at QAlb = 6.5 × 10−3 (≤ 40 years), and at QAlb = 8 × 10−3 (≤ 60 years). Values below the lower hyperbolic line in range 5 indicate a faulty method.22,23
Patterns of synthesis of intrathecal immunoglobulins and complement components C3c and C4 in angiostrongyliasis.
Different patterns of intrathecal immunoglobulin synthesis have been described.24 Diagnostic examination of CSF from the lumbar puncture shows no intrathecal IgG, IgA, or IgM class response. A week later, at the time of early clinical recovery, intrathecal immunoglobulin synthesis has emerged as either a two-class or three-class response.
Intrathecal synthesis patterns and their frequency are shown in Table 1. Pattern 1 is the most frequent and includes the major complement components C3c and C4. Intrathecal synthesis of at least one of the major immunoglobulins may be observed in these patients; a wide spectrum of patterns may occur. As might be expected, the patients have C3c intrathecal synthesis; C4 intrathecal synthesis was noted in some patients. The C3c and C4 Reibergrams from patients with angiostrongyliasis are shown in Figures 1 and 2, respectively.
Table 1.
Synthesis patterns and frequency of patients with angiostrongyliasis
| Pattern | Synthesis pattern | Frequency |
|---|---|---|
| I | IgA + IgG + IgM + C3c + C4 | 4/20 |
| II | IgA + IgG + IgM + C3c | 3/20 |
| III | IgA + IgM + C3c | 1/20 |
| IV | IgA + IgM + C3c + C4 | 2/20 |
| V | IgA + IgG + C3c + C4 | 2/20 |
| VI | IgG + IgM + C3c + C4 | 1/20 |
| VII | IgM + C3c | 3/20 |
| VIII | IgA + C3c | 2/20 |
| IX | C3c | 2/20 |
General intrathecal synthesis variants.
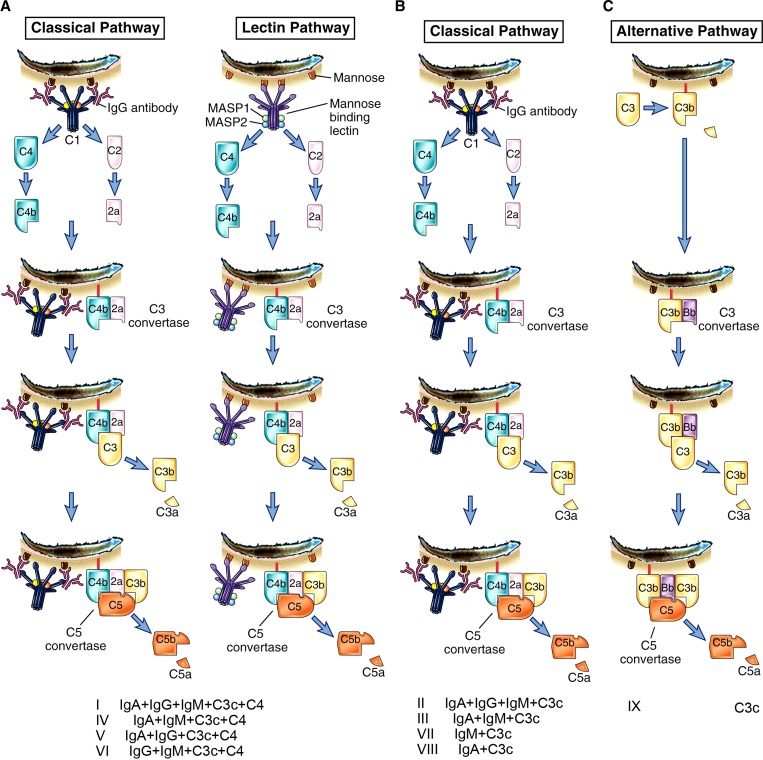
Diversity of intrathecal synthesis and activation of different complement pathways enables their division into three variant groups (A, B, and C) (Figure 4). Samples of CSF and serum were obtained from patients diagnosed with A. cantonensis eosinophilic meningitis. The patients had typical symptoms of meningitis. Samples obtained at the time of admission (at the onset of symptoms) were kept in small aliquots at –80°C until analysis. All data were stored in historical records.
Figure 4.
General intrathecal synthesis variants of intrathecal patterns. Four pathways are shown.
Variant A.
This variant includes classical and/or lectin pathways and generally involves ≥ 2 major immunoglobulins with intrathecal synthesis of C3 and C4. Patterns I, IV, V, and VI were observed among this variant. Pattern I is intrathecal synthesis of major immunoglobulins with C3 and C4 plus local synthesis. The classical and/or lectin pathway (intrathecal activation) may be observed and have a potential role in the destruction of third-stage parasite larvae. Pattern IV is intrathecal synthesis of IgA and IgM simultaneously with C3 and C4 local synthesis involved in classical and/or lectin pathway (intrathecal activation). Pattern V is intrathecal synthesis of IgA and IgG simultaneously with C3 and C4 local synthesis, acting as classical and/or lectin pathway (intrathecal activation). Pattern VI is intrathecal synthesis of IgM and IgG simultaneously and can produce classical and/or lectin intrathecal activation with C3 and C4 intrathecal synthesis.
Variant B.
All responses classified in this variant group are characterized by C4 in CSF that comes from blood in the intrathecal activation of the classical pathway. This group includes patterns II, III, VII, and VIII. Pattern II is classical intrathecal activation by IgG and IgM, and occasionally IgA1 may act by fixing complement with C3 intrathecal synthesis. Pattern III is classical intrathecal activation by IgM and occasionally by IgA1 with C3 intrathecal synthesis. Pattern VII shows efficient fixation of complement by IgM and intrathecal activation of C3 by the classical pathway. Pattern VIII shows intrathecal activation by a classical pathway caused by C3c and IgA1 synthesized locally.
In patients who show major immunoglobulin intrathecal synthesis but no C4 intrathecal synthesis, unique activation might be the classical pathway. However, C4 originates from blood by simple diffusion.
Variant C.
This variant only includes the alternative pathway. There is only intrathecal synthesis of C3 (pattern IX). The patient included in this group show only C3c intrathecal synthesis, and the only complement pathway observed is the alternative pathway. In this pathway, the interaction among C3, properdin, and B and D factors facilitate C3bBbP complex formation, and activity against and lysis of larvae. Until now, there has been no report of C3 deficiency in patients with eosinophilic meningoencephalitis caused by A. cantonensis.
In the genetic absence of C3, thrombin substitutes for C3-dependent C5 convertase. This linkage between the complement and coagulation pathways may represent a new pathway of complement activation.27 Thrombin can function as C5 convertase and provides evidence for a direct linkage between complement and clotting pathways. This finding suggests a new pathway of complement activation in the absence of C3. This recently described pathway of complement should be taken into account in future studies.
Pattern IX is characterized by intrathecal activation of the alternative pathway and absence of intrathecal synthesis of immunoglobulins. This finding suggests that intrathecal immunoglobulins are not involved in local activation of the complement system. Other complement pathways, should they be activated, will have been produced by immunoglobulins from blood.
Role of complement intrathecal activation in larval destruction.
Two processes involving complement occur separately, but have an intimate relationship: biological synthesis and activation. In blood, these processes also occur separately. However, in blood they constitute a system with many possibilities. These possibilities include supplying components to different pathways capable of acting against a wide range of invading organisms. These complement components can be used variably against different microorganisms as part of the innate immune response. Complement components of different pathways act as acute-phase proteins and participate in the initial host response before immunoglobulin production.
Third-stage larvae are usually destroyed in human CSF, in which case the complement system may play an important role. To understand this local process, it is important to clarify the meaning of intrathecal activation. This term can be defined as the activation of any complement pathway mediated by one or more of the components of the complement system previously synthesized in CSF. It is important to note that intrathecal activation does not exclude the possibility that the complement system can be activated by components in blood. However, this activation is not a direct consequence of the presence of third-stage larvae of A. cantonensis in CSF.
Intrathecal immune response patterns and consequences for blood-CSF barrier function, combined with synthesis and activation of the complement system caused by parasites, have not been reviewed. These patterns are discussed for pathophysiologic (diagnostic and theoretical) reasons. The main feature of intrathecal activation is that it is a more specific response that occurs in CSF, in which third-stage larvae are inactivated, and in which an inflammatory response is produced. These responses are part of the process of elimination of parasites in this environment. It is noteworthy that the role of complement and its role in in vivo during helminth infection is poorly understood.28 In the absence of intrathecal activation, any larvae that had evaded destruction in the blood, could migrate to the CNS. Thus, the complement system could be one of the most important elements in the brain's primary defense against infections, especially early when specific antibodies have not been synthesized.
Although immunoglobulins commonly pass from the blood to the CSF, it is nevertheless important to recognize the existence of local synthesis and the intrathecal immune response, and to determine the role played by these immunoglobulins against third-stage larvae in situ. In addition, local complement synthesis in the CSF is proof of production and intrathecal activation of C3 from its degradative derivative C3c.
Peculiarities of the immune response in CNS.
The humoral immune response in CNS differs from the immune response seen in blood. Importantly, in the CNS, there is no switch from an IgM class response to a more specific IgG class response in the course of inflammatory neurologic disease.29,30 The pattern of intrathecal IgG/IgA/IgM synthesis remains rather constant and depends on the cause, pathophysiology, and localization of the disease process.
Lack of an IgM to IgG switch and slow normalization of intrathecal antibody synthesis is regarded as consequences of the specific regulation of the intrathecal immune response.31,32 From a diagnostic point of view, the lack of an IgM to IgG switch in CNS enables one to characterize disease-related patterns instead of acuity-related patterns. In the special environment of the CNS, there are other peculiarities in the immune response.
In addition to the absence of a switch from IgM to IgG that is widely used for diagnostic purposes,30,31 regulation of the immune response shows other differences. One fact to be taken into account is that existence of immunoglobulins intrathecally synthesized does not preclude the possibility that some of these CNS immunoglobulins may have reached the CSF from blood. It is possible to differentiate the fraction of the brain-derived local synthesis by using a Reibergram.29
In eosinophilic meningitis caused by A. cantonensis, CNS damage caused by the motile worm, inflammatory responses to foreign antigens, and possible toxicity of worm components work in concert to produce the pathology and clinical disease picture. The immune response in this disease, as detected in the CSF, clearly shows these pathologic changes and clinical findings.
The classical and lectin pathways of complement activation can lead to the inflammatory process during meningitis. C4 can play an essential role by interaction with mannose-binding lectin or C2 from the classical pathway.33 Neurotoxins produced by eosinophils during the inflammation process, including antibody production, should be the primary causes of CNS damage. C4 with immunoglobulin intrathecal synthesis occurs at least by the classical pathway. Antibody-dependent complement cytotoxicity may then occur. This type of cytotoxicity, rather than phagocytosis,32 may eliminate A. cantonensis larvae.
Patients with angiostrongyliasis can be expected to manifest synthesis of C3c as a degradation product of C3. First, this expectation indicates that complement activation has occurred by at least one of the three pathways and that the involved protein was biologically used. Second, when the role of C3 is completed, it was modified into C3c in the CSF. Studies have indicated that C3 is almost exclusively responsible for facilitating the stable attachment of eosinophils and other leukocytes to the specific pathogen.34
The practical use of C3c as a split fraction of C3 intrathecal synthesis is a measure of the functionality of this fraction during intrathecal activation. It is also a sensitive marker of complement system activation in general. Patterns that involve C4 intrathecal synthesis always include C3c intrathecal synthesis, and provide additional information about the biological process that generally includes IgM intrathecal synthesis. All of these patterns enable a more complete picture of this important process, which can clarify the mechanism of the observed lysis of third-stage larvae of A. cantonensis in the CSF.
This review of the process of intrathecal inactivation during angiostrongyliasis has included those facts that we believe are most relevant. The significance of some of the observations reported is presently unknown. These observations include the role of the complement system in destruction of larvae. An understanding of this process could facilitate strategies for anti-parasitic drug design. These findings have led us to make two conclusions. First, complement is a competitive natural adjuvant, and an understanding of its manifold pathways will ultimately enable understanding of angiostrongyliasis. Second, this understanding should identify the best way to eliminate invasive larvae, even before their entry into the CNS.
ACKNOWLEDGMENTS
We thank Professor Dr. Ernest A. Meyer for reviewing the manuscript and Manuel Rodriguez for graphic design. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Authors' addresses: Barbara Padilla-Docal, Ivonne Iglesias-González, Raisa Bu-Coifiu-Fanego, Carmen Aleida Socarrás-Hernández, and Alberto Juan Dorta-Contreras, Facultad de Ciencias Médicas Dr. Miguel Enríquez – Investigaciones, Ramón Pintó 202 Luyanó, Havana 10700, Cuba, E-mails: barbara.padilla@infomed.sld.cu, iiglesias@infomed.sld.cu, raisabu@infomed.sld.cu, carmen.hdez@infomed.sld.cu, and dorta@infomed.sld.cu.
References
- 1.Wang QP, Lai DH, Zhu XQ. Human angiostrongyliasis. Lancet Infect Dis. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman S. Eosinophilic meningitis due to Angiostrongylus cantonensis in a returned traveler: case report and review of literature. Clin Infect Dis. 2001;33:12–15. doi: 10.1086/323460. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Feng X, Jin-Bao G, Xiao-Guang C. Case report: a severe eosinophilic meningoencephalitis caused by infection of Angiostrongylus cantonensis. Am J Trop Med Hyg. 2008;79:568–570. [PubMed] [Google Scholar]
- 4.Lindo JF, Waugh C, Hall J, Cunningham-Myrie C. Enzootic Angiostrongylus cantonensis in rats and snails after an outbreak of human eosinophilic meningitis, Jamaica. Emerg Infect Dis. 2002;8:324–326. doi: 10.3201/eid0803.010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorta-Contreras AJ, Padilla-Docal B, Moreira JM. Neuroimmunological findings of Angiostrongylus cantonensis meningitis in Ecuadorian patients. Arq Neuropsiquiatr. 2011;69:466–499. doi: 10.1590/s0004-282x2011000400011. [DOI] [PubMed] [Google Scholar]
- 6.Padilla-Docal B, Dorta-Contreras AJ, Moreira JM. Comparison of major immunoglobulins intrathecal synthesis patterns in Ecuadorian and Cuban patients with angiostrongyliasis. Am J Trop Med Hyg. 2011;84:406–410. doi: 10.4269/ajtmh.2011.10-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen L, Laigret J, Boils PL. Observation on an outbreak of eosinophilic meningitis on Tahiti, French Polynesia. Am J Hyg. 1961;74:26–42. doi: 10.1093/oxfordjournals.aje.a120198. [DOI] [PubMed] [Google Scholar]
- 8.Kliks MM, Kroenke K, Hardman JM. Eosinophilic radiculomyeloencephalitis: an angiostrongyliasis outbreak in American Samoa related to ingestion of Achatina fulica snails. Am J Trop Med Hyg. 1982;31:1114–1122. doi: 10.4269/ajtmh.1982.31.1114. [DOI] [PubMed] [Google Scholar]
- 9.Chen WL, Zhong JM, Chen H, Wu SY, Ding L. Eosinophilic meningitis: 31 cases report. Chin J Misdiagn. 2006;6:4668–4669. [Google Scholar]
- 10.Pascual Gispert JE, Aguilar Prieto PH, Galvez Oviedo MD. Finding of Angiostrongylus cantonensis in the cerebrospinal fluid of a boy with eosinophilic meningoencephalitis. Rev Cubana Med Trop. 1981;33:92–95. [PubMed] [Google Scholar]
- 11.Slom TJ, Cortese MM, Gerber SI. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med. 2002;346:668–675. doi: 10.1056/NEJMoa012462. [DOI] [PubMed] [Google Scholar]
- 12.Graeff Texeira C. Expansion of Achatina fulica in Brazil and potential increased risk for angiostrongyliasis. Trans R Soc Trop Med Hyg. 2007;101:743–744. doi: 10.1016/j.trstmh.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Leone S, De Marco M, Ghirga P. Eosinophilic meningitis in a returned traveler from Santo Domingo: case report and review. J Travel Med. 2007;14:407–410. doi: 10.1111/j.1708-8305.2007.00152.x. [DOI] [PubMed] [Google Scholar]
- 14.Pincay T, García L, Narváez E. Angiostrongyliasis due to Parastrongylus (Angiostrongylus) cantonensis in Ecuador. First report in South America. Trop Med Int Health. 2009;14:37. [Google Scholar]
- 15.Abbas AK, Lichtman AH, Pillai S. Innate immunity. In: Abbas AK, Lichtman AH, Pillai S, editors. Cellular and Molecular Immunology. Philadelphia, PA: Saunders; 2011. pp. 56–88. [Google Scholar]
- 16.Garred P, Honoré C, Ma Ying J. MBL2, FCN1, FCN2 and FCN3: the genes behind the initiation of the lectin pathway of complement. Mol Immunol. 2009;46:2737–2744. doi: 10.1016/j.molimm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Ramos TN, Darley MM, Weckbach S, Stahel PF, Tomlinson S, Barnum SR. The C5 convertase is not required for activation of the terminal complement pathway in murineexperimental malaria. J Biol Chem. 2012;287:24734–24738. doi: 10.1074/jbc.C112.378364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorta-Contreras AJ. Reibergrama como herramienta epidemiológica: nuevo enfoque. Rev Neurol. 2001;33:36–40. [PubMed] [Google Scholar]
- 19.Dorta-Contreras AJ. Pattern of intrathecal immunoglobulin synthesis in pediatric patients with infectious meningoencephalitis. Biotecnol Apl. 2006;4:382–386. [Google Scholar]
- 20.Dorta-Contreras AJ, Noris-García E, Padilla-Docal B. Reibergrama para la evaluación de la síntesis intratecal de C3c. Arq Neuropsiquiatr. 2006;64:586–588. doi: 10.1590/s0004-282x2006000400010. [DOI] [PubMed] [Google Scholar]
- 21.Padilla-Docal B, Dorta-Contreras AJ, Bu-Coifiu-Fanego R, Ray R. CSF/serum quotient graphs for the evaluation of intrathecal C4 synthesis. Cerebrospinal Fluid Res. 2009;6:8. doi: 10.1186/1743-8454-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorta Contreras AJ, Reiber H. Teoría de la difusión molecular/flujo del líquido cefalorraquídeo. Rev Neurol. 2004;39:564–569. [PubMed] [Google Scholar]
- 23.Reiber H. Flow rate of cerebrospinal fluid (CSF): a concept common to normal blood–CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. 1994;122:189–203. doi: 10.1016/0022-510x(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 24.Dorta-Contreras AJ, Reiber H. Intrathecal synthesis of immunoglobulins in eosinophilic meningoencephalitis due to Angiostrongylus cantonensis. Clin Diagn Lab Immunol. 1998;5:452–455. doi: 10.1128/cdli.5.4.452-455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorta-Contreras AJ, Noris-García E, Escobar-Perez X, Padilla-Docal B. IgG1, IgG2 and IgE intrathecal synthesis in Angiostrongylus cantonensis meningoencephalitis. J Neurol Sci. 2005;238:65–70. doi: 10.1016/j.jns.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Padilla-Docal B, Dorta-Contreras AJ, Bu-Coifiú Fanego R, Hernández HF, Barroso JC, Sanchez-Martinez RC. Intrathecal synthesis of IgE in children with eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis. Cerebrospinal Fluid Res. 2008;5:18. doi: 10.1186/1743-8454-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber-Lang M, Vidya Sarma J, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flieri MA, Hoesel LM, Gebhard F, Younger JD, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 28.Giacomin PR, Wang H, Gordon DL. Loss of complement activation and leukocyte adherence as Nippostrongylus brasiliensis develops within the murine host. Infect Immun. 2005;73:7442–7449. doi: 10.1128/IAI.73.11.7442-7449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorta-Contreras AJ. Dinámica de la síntesis intratecal de inmunoglobulinas. Rev Neurol. 2000;31:991–993. [PubMed] [Google Scholar]
- 30.Reiber H. Quantitative Proteinanalytik, Quotientendiagramme und krankheitsbezogene Datenmuster. In: Zettl UK, Lehmitz R, Mix E, editors. Klinische Liquordiagnostik. Berlin: Walter de Gruyter & Co.; 2003. pp. 177–200. [Google Scholar]
- 31.Reiber H. Neuroimmunology: immunoglobulins and the intrathecal polyspecific immune response in acute, subacute and chronic neurological disease. J Int Fed Clin Chem LabMed. 2004;15 www.ifcc.org/ejifcc/vol15no3/150309200412.htm Available at. [PMC free article] [PubMed] [Google Scholar]
- 32.Dorta-Contreras AJ, Núñez-Fernández FA, Pérez-Martín O. Peculiaridades de la meningoencefalitis por Angiostrongylus cantonensis en América. Rev Neurol. 2007;45:755–763. [PubMed] [Google Scholar]
- 33.Padilla-Docal B, Dorta-Contreras AJ, Bu-Coifiu-Fanego R, Rodríguez-Rey A, Gutiérrez-Hernández JC, de Paula-Almeida OS. Reibergram of intrathecal synthesis of C4 in patients with eosinophilic meningitis caused by Angiostrongylus cantonensis. Am J Trop Med Hyg. 2010;82:1094–1098. doi: 10.4269/ajtmh.2010.09-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padilla-Docal B, Dorta-Contreras AJ, Bu-Coifiu-Fanego R. Activación y biosíntesis intratecal de C3c en niños con meningoencefalitis eosinofílica por Angiostrongylus cantonensis. Rev Neurol. 2009;48:632–635. [PubMed] [Google Scholar]